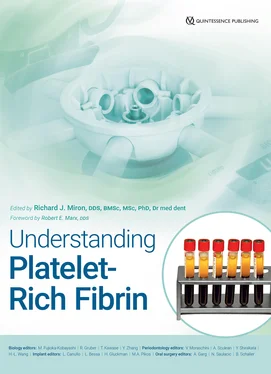
Figure 3-16demonstrates the overall final volume of plasma of each of the protocols, along with the total yield and concentrations of platelets and leukocytes above baseline values. Note that of each of the protocols, 200g for 5 minutes led to the highest concentration of platelets/leukocytes. The best yield of leukocytes was achieved after centrifugation for 8 minutes utilizing the 700g, 1000g, and 1200g protocols. Of those, the highest concentration was achieved at 700g for 8 minutes (owing to the reduced total plasma volume). Another noteworthy trend that was apparent was that as centrifugation time was increased, a general increase in percent yield was observed; however, generally speaking, an overall decrease in concentration was also observed (see Fig 3-16). Furthermore, it was apparent that certain centrifugation protocols that were too reduced in RCF (such as 100g) typically did not lead to adequate yield of cells. This relates with our group’s previous work on i-PRF demonstrating that these low centrifugation speeds and time (~800 rpm for 3–4 minutes) led to substandard concentrations of platelets and leukocytes (see chapter 2). Protocols that were too fast or lengthy (1000g or more) led to a reduction in yield and/or concentrations (as more cells then got pushed into the bottom layers or the volume of total plasma led to a reduction in concentration).
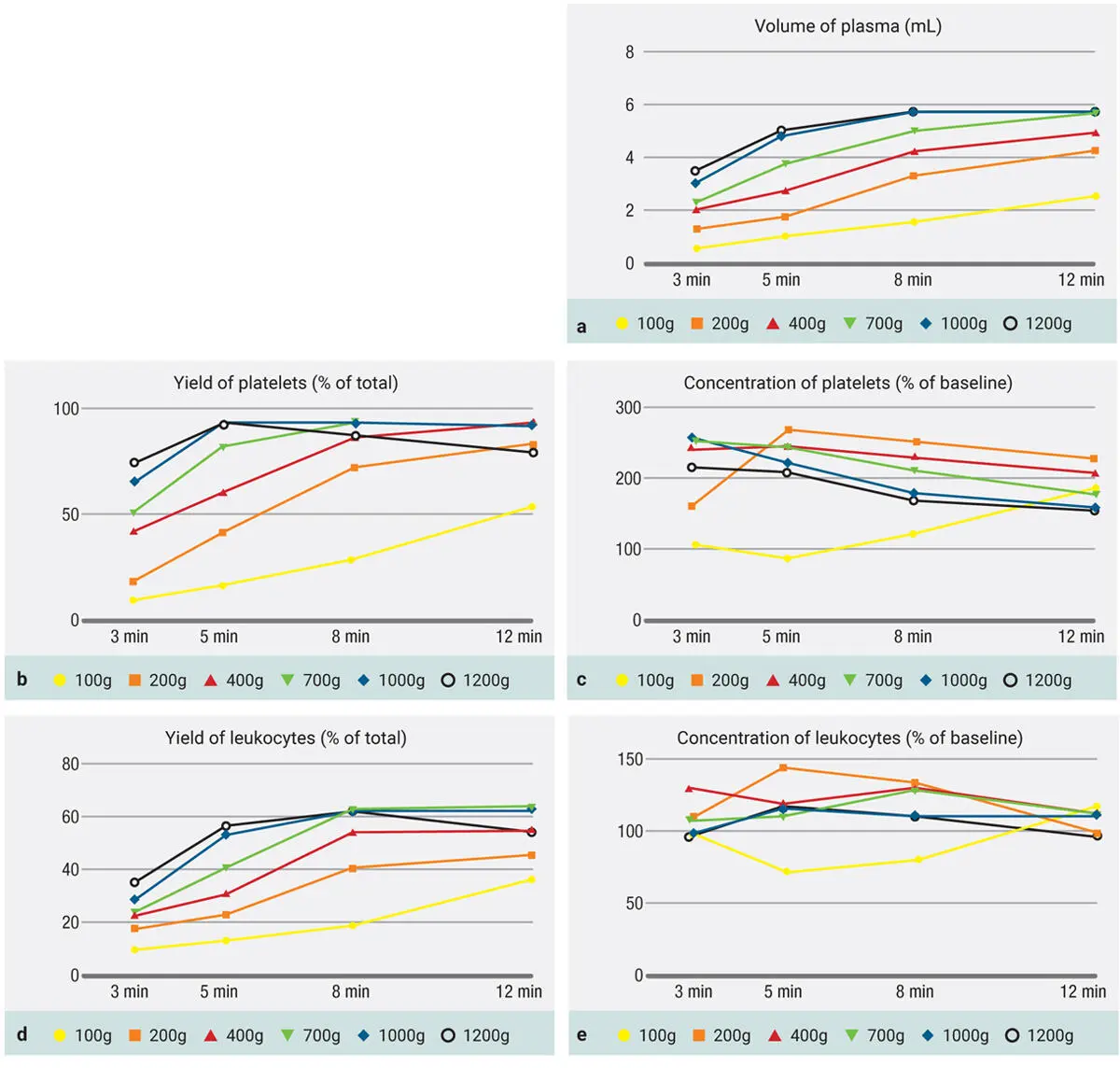
Fig 3-16 (a to e) Evaluation of 24 protocols utilized for the production of PRF. Data includes final volume (mL), total leukocyte and platelet yields (% of the total from 10 mL), as well as concentration of leukocytes and platelets above baseline values (% increase). (Reprinted with permission from Miron et al. 8)
To simplify Fig 3-16, certain time points were removed from the graph to facilitate its understanding for the reader. Figure 3-17demonstrates only two protocols (700g and 1000g) over time. Note that in Fig 3-17a, a general increase of centrifugation time is associated with an increase in platelet yield. Note, however, in Fig 3-17b that an increase in centrifugation time actually decreases the concentration of platelets (because the plasma volume is increased, so even if the total yield of platelets remains the same or even slightly higher, the actual concentration decreases).
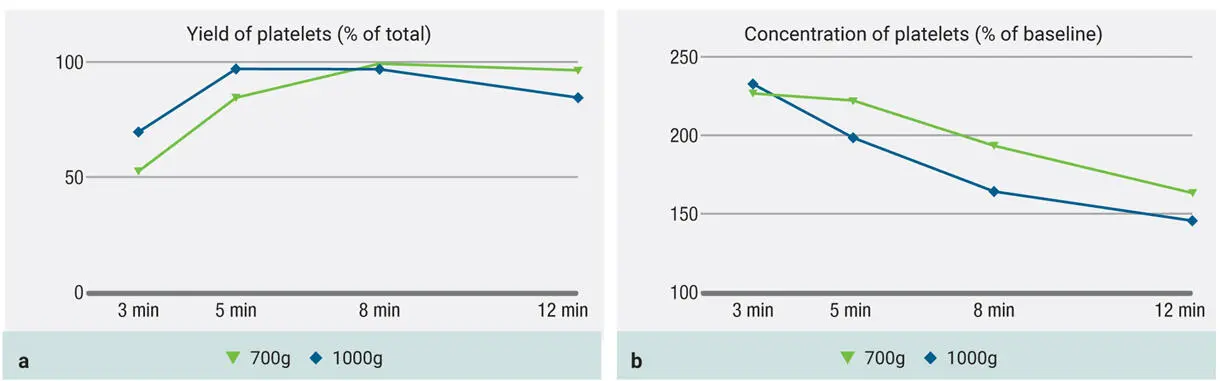
Fig 3-17Evaluation of protocols utilizing both 700g and 1000g RCF for the production of PRF. Both total yield of platelets (a) as well as concentration of platelets (b) are depicted. (Reprinted with permission from Miron et al. 8)
In Fig 3-18, the 100g protocol is included as well. Notice here how the yield is extremely low in platelets ( Fig 3-18a) as well as in concentration ( Fig 3-18b). This is a result of the speed cycle simply being so slow that it is unable to accumulate or concentrate platelets in the upper layer. As alluded to in chapter 2, it is possible to centrifuge too slowly to the point where platelets and leukocytes do not actually accumulate effectively in the upper layers. This is a common misconception that many clinicians maintain due to inaccurate information provided by various manufacturers.
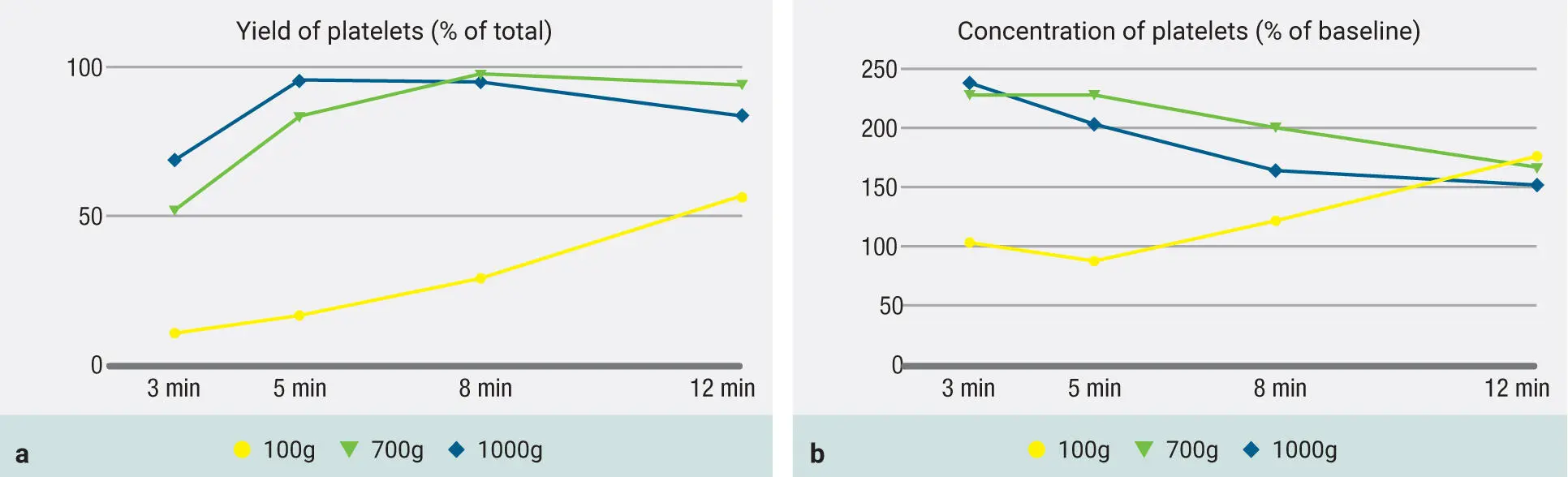
Fig 3-18Previous graphs demonstrating 700g and 1000g protocols but with the addition of 100g results as well. It is very clear that the 100g protocol is not able to accumulate high yields of platelets (a) , and the concentration remains low (b).
Centrifugation carried out too slowly is a common misconception that many clinicians maintain due to inaccurate information provided by various manufacturers.
In Fig 3-19, observe the 200g protocol. Notice how at the higher g-force, the cells are actually able to accumulate more efficiently ( Fig 3-19a). More importantly, observe the concentration of platelets in Fig 3-19b following a 200g protocol for 5 minutes. Here the platelets are actually most concentrated after 5 minutes, and thereafter, even though their yield continues to rise (see Fig 3-19a), the concentration actually begins to decrease because of the increase in liquid-PRF volume. Therefore, a 200g to 300g centrifugation cycle is most effectively able to concentrate platelets and leukocytes (300g is the one chosen after further optimization).
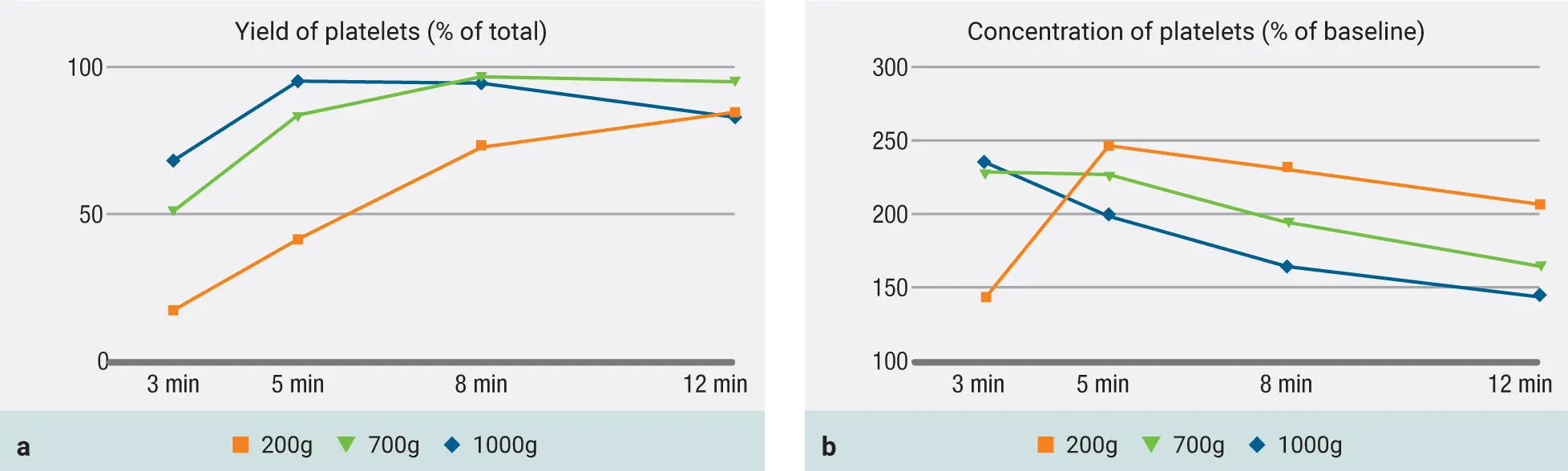
Fig 3-19Evaluation of protocols utilizing both 700g and 1000g RCF for the production of PRF but now with the addition of the 200g protocols. Both total yield of platelets as well as concentration of platelets are depicted. Note that when a 200g protocol is utilized, while the yield is still low (a) , the concentration peaks at 5 minutes at a level higher than any other group (b) . (Reprinted with permission from Miron et al. 8)
Discussion
In general, platelets were evenly distributed throughout a variety of protocols within the upper three to six plasma-rich layers (see Fig 3-16), but it was obvious that WBCs required more pristine fine-tuning to reach adequate harmony within the upper plasma layers. Protocols within the 400g to 700g (5- to 8-minute) range were better able to accumulate and distribute platelets/leukocytes more evenly throughout the upper layers.
Currently, one standard in the field of PRF is the novel use of injectable i-PRF. 9Previously, our research group found that only slight increases in platelets and leukocytes were noted, with failure to adequately accumulate cells in the upper plasma layer owing to extremely low RCF values (60g) and centrifugation times (3–4 minutes) on fixed-angle centrifugation devices. 1In a study titled “Injectable platelet-rich fibrin: Cell content, morphological, and protein characterization,” 10Varela et al observed only a slight increase in platelets (less than 33%) and leukocytes following i-PRF protocols, with decreases in VEGF reported when compared to that in whole blood. Altogether, these studies confirm that previously utilized i-PRF protocols (~60g for 3–4 minutes on a fixed-angle centrifuge) are inadequately effective at separating blood cell layers due to their considerable reduction in centrifugation speed and time. Furthermore, protocols at 100g or lower are inefficient at accumulating platelets and leukocytes in the upper plasma layer, highlighting the limits of the LSCC.
It was also observed within this study that the use of PRF produced using a protocol of 200g for 5 minutes resulted in the highest concentration of platelets and leukocytes. Within these studies, up to a fourfold increase in platelet/leukocyte concentration and/or yield was observed when compared to the results of previously utilized i-PRF protocols produced on a fixed-angle centrifuge. 1Further unpublished data has found that a 300g protocol for 5 minutes resulted in the highest concentration of liquid-PRF, as discussed further in chapter 5. Nevertheless, based on the C-PRF protocols established in chapter 2, it remained obvious that better concentrations could be achieved by further modifying centrifugation parameters.
Establishing C-PRF on a Horizontal Centrifuge
In a recent study titled “Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared to traditional injectable (i-PRF) protocols,” Fujioka-Kobayashi et al aimed to investigate and optimize PRF in its most concentrated formulation. 11As reviewed in chapter 2, the ability to centrifuge at higher centrifugation speeds and times leads to an accumulation directly at the buffy coat layer. 12There was an approximately tenfold increase in baseline concentrations specifically in this 0.3- to 0.5-mL buffy coat layer directly above the RBC layer produced using higher centrifugation protocols. 12The PRF obtained from this harvesting technique was given the working name concentrated-PRF (C-PRF). Figure 3-20demonstrates a clinical photograph of standard liquid-PRF protocols versus those of C-PRF. It was hypothesized that based on the extensive increase in the yield of platelets and leukocytes, C-PRF would exhibit higher GF release as well as superior cellular activity. Therefore, the aim of this study was twofold. First, a new centrifugation protocol was developed on a horizontal centrifugation system with the aim of accumulating the greatest concentrations of platelets and leukocytes within the buffy coat. The second aim was to compare the total GF release of PRF obtained through this newly developed C-PRF protocol to that of PRF obtained through the clinically utilized liquid i-PRF protocol over a 10-day period and to investigate the regenerative properties of human gingival fibroblasts in vitro.
Читать дальше