Applied Soil Chemistry
Здесь есть возможность читать онлайн «Applied Soil Chemistry» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Applied Soil Chemistry
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Applied Soil Chemistry: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Applied Soil Chemistry»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Applied Soil Chemistry — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Applied Soil Chemistry», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Most biomass consists of complex mixtures of the organic materials mentioned all decaying at different rates and forming physical and chemical mixtures. The presence of lignin in such complex mixtures tends to slow down the decomposition of the components that on their own would decay more rapidly. The degradations of mixtures of organic materials containing cutins and tannins can also be retarded in a similar manner.
Microbes constitute up to about 3% of the organic matter in soils. Bacteria and fungi have their own complex chemical makeups that distinguish them from plant and animal derived biomass. Melanins, derived from some fungi, are not degraded easily and are partly responsible for the dark coloration of some soils. Microbial actions tend to remove visual signs of the structures of organisms they decay turning the organic material into a biochemical soup revealing only their microbial provenance. Some non-biological synthesis of chemical bonds does occur in this biochemical soup, in the presence of oxygen and groundwater of varying acidity, contributing to the humification of soils. The humic materials generated to be stable and not susceptible to fragmentation by biologically produced enzymes [17]. During decomposition the carbon quantity of the organic materials in soils increases from about 40% (undecayed plant material) to about 60% (fully humified soil). Humified soil is not just organic material, it involves mixtures with various inorganic minerals from the weathered outcropping rocks on which it resides, particularly the finer grained clay minerals. The combination of decayed organic material with inorganic minerals, physically as grain coating and chemically as mineralization reactions, aids the structural integrity of soils and often helps to inhibit mechanical processes involved in soil erosion.
1.1.3 Cycle Time of Carbon in Soils
Despite a global balance between carbon inflow from biological sources to a soil and CO 2output to the atmosphere through soil respiration, that process tends not to be a smooth sequential progression when observed locally in specific soils. On the contrary, carbon introduced in one seasonal cycle may take multiple seasonal and/or annual cycles before it is decayed step-by-step and ultimately becomes mineralized. The CO 2soil respiration output at any point in time comes cumulatively from organic material, at various stages of decay, introduced to the soil during multiple seasonal and annual cycles. The turnover time for carbon in soils varies significantly depending on its stage of maturity and can be determined precisely by carbon isotope dating ( 14C) techniques. For immature soils, rich in recently demised and introduced biological material, the turnover time is no more than 5 years and could be less than one year. For mineralized soil mixed with inorganic minerals, the turnover time is likely to amount to several decades. The most mature humified soils, from which there is very little carbon inflow and outflow, the carbon turnover times can be measured in thousands of years, meaning that they represent almost inert systems from the perspective of carbon flow [18].
It is often useful to establish turnover times for specific soils. In practice, individual soils will contain components and/or layers displaying a wide range of carbon turnover times. The turnover time of a particular soil layer tends to be influenced by the vegetative geographic zone which determines its sustained humidity and temperature and nutrient content. However, soil instability, due to impacts of extreme seasonal climatic swings and/ or severe weather events that can frequently disturb soils, for example, by increasing leaching rates by ground water, do substantially influence carbon turnover times in some cases.
He et al . (2016) [19] presented 14C dating measurements on 157 globally distributed soil profiles sampled to 1-m depth. Their results revealed that most existing Earth system models tended to substantially underestimate the mean age of carbon in all common soil types. Moreover, that underestimate was more than six-fold (430 ± 50 years versus 3,100 ± 1,800 years). This finding is not good news for soils as a potential carbon sequestration store, implying that models were making two-fold overestimates of soils global carbon sequestration capabilities. The results indicate that carbon stabilization is a slow process in many soils with long turnover times. This makes soils relative slow-to-develop and passive reservoirs, except on geological time scales, meaning that they cannot absorb large quantities of carbon over short periods of time. This limits soils potential to be a major part of a short-term carbon sequestration solution aimed at removing carbon from the atmosphere on a timescale of decades and over the duration of coming century, which is required to rapidly reverse rising carbon levels in the atmosphere.
The UN’s Intergovernmental Panel on Climate Change (van Diemen, 2019) [20], among other bodies (e.g., Halldorsson et al ., 2015 [21]), had previously developed its models for carbon sequestration by soils assuming substantially shorter carbon cycling times. This erroneous assumption led the IPCC to suggest if deforestation on a global scale could be halted about 40 ppm of CO 2, about 10% of current levels could be sequestered into soils from the atmosphere. It was believed that combined with major global changes in agricultural practices even more carbon could be absorbed by soils. There is now a more realistic recognition that the carbon absorption ability soils and their turnover periods for carbon cannot be substantially increased in the short-term from the prevailing slow rates [19].
1.2 Influences Impacting Carbon Stabilization Rates in Soils
The amount of organic material present in soils is influenced by many factors ( Figure 1.2). Establishing knowledge and understanding of the key influencing factors are essential in determining how carbon uptake and retention of certain soil types can be improved from a long-term carbon capture and sequestration perspective. In a specific soil, the alteration rate of the unit quantity of organic material it contains is established by subtracting the degradation rate from the input rate of biologically derived material. The organic matter degradation rate is positively correlated with the quantity of organic matter present in the soil. This relationship means that progressively the degradation rate converges to the input rate as a soil matures to establish an environmentally adjusting equilibrium with the organic matter remaining constant from that point. The quantity of carbon resident and fixed in a unit mass of soil can therefore be enhanced by either reducing the rate of its degradation of organic matter or accelerating the input of organic matter. On the other hand, more carbon can also be fixed in a soil system if more mature humified material is continuously produced. This is because the humified material tends to be inert with carbon contributing to its formation, but unlikely to leave it for millennia until it is eroded by geological processes or excavated by anthropogenic activities.
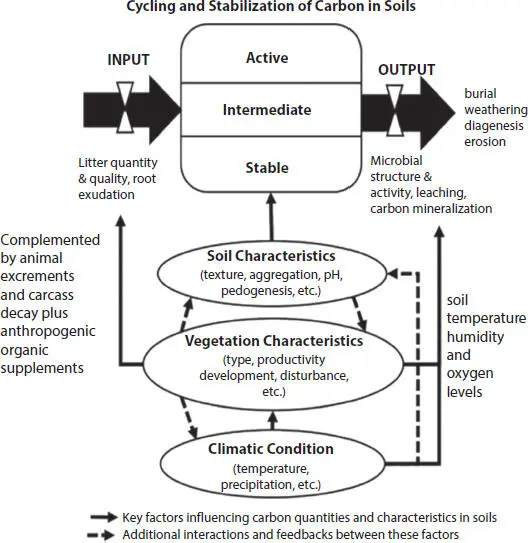
Figure 1.2 Schematic diagram showing how the key variables of climate, vegetation, and soil characteristics impact organic matter concentrations in soils (modified after Sun et al ., 2019 [22], who developed the diagram with a specific focus on forest ecosystems).
1.2.1 Weather Conditions and Fluctuations
Prevailing weather conditions substantially influence the quantity of carbon present in soils. This is not simply due to differences in specific climatic zones and geographical environments that clearly plays a substantial role. It is well established that the carbon content of soils reduces from the equator to the poles, mainly as a consequence of climatic variations [23]. Specifically, it is biophysical variables such as humidity and temperature conditions in a soil that determine the rates of microbial and bacterial metabolism. Up to a point higher temperatures and humidity tend to increase metabolic rates; whereas, water-saturated soils, starved of oxygen result in a decrease in metabolic rates. It is the latter conditions that are responsible for massive deposits of peat, formed in cooler and wetter regions and tropical regions, resulting in massive carbon sinks developed over millennia. In many environments, soil water content is the dominant factor in determining the quantity of carbon preserved in peats. In cool or warm arid environments, the lack of soil moisture tends to inhibit the degradation of organic matter but carbon input tends to be low in such soils, leading to their low NPP.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Applied Soil Chemistry»
Представляем Вашему вниманию похожие книги на «Applied Soil Chemistry» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Applied Soil Chemistry» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












