On a global scale, soils store about 1,500 Pg (petagrams, equivalent to 1,500 gigatonnes) of organic carbon to a depth of one meter, increasing to 2,400 Pg to a depth of 2 m [11]. This means that carbon residing in upper soil layers amounts to more than the combined quantity of carbon in land-surface vegetation and the atmosphere. A little less than 50% of soils globally have been or are in use for agriculture, both cropland and grazing land. The soils involved in cropland activity have almost all been disturbed by some form of tillage. Organic matter within soils can vary between about 1% and 10%.
Subsequently, that carbonic acid reacts with basic cations leading to the creation of secondary carbonates in the short-term, on the scale of years, forming mineralization in near-surface rocks, leading to sustained processes that persists over geological timescales. The creation of secondary carbonates comes mainly from sub-surface weathering and diagenesis reactions with silicate minerals containing calcium and magnesium. Such reactions generate free positively charged ions (cations). Many of these free cations go on to combine with CO 2to form carbonate minerals, particularly calcite and dolomite [12]. However, these pervasive carbonate forming diagenetic processes tend to progress too slowly in their natural cycles to be practically exploited for carbon sequestration purposes. Nevertheless, they do involve substantial quantities of CO 2, particularly in alkaline and saline soils present in dry and semi-dry zones [13]. Consequently, the inorganic sub-surface carbon cycle cannot be considered as significant or viable for rapid carbon sequestration in the soils typically found in the soils of wet and temperate zones.
On the other hand, organic carbon can cycle through soils, some returning to the atmosphere very rapidly. In the organic dimensions of the carbon cycle, atmospheric carbon dioxide is stabilized through the photosynthesis conducted by plants, algae, and cyanobacteria to form a range of organic compounds. Although the living organisms initially form glucose during photosynthesis, they transform it into diverse organic compounds, such as cellulose, hemicellulose, and lignin mostly; materials that are useful for biological growth and tissue formation. However, other complex organic materials such as protein, lipids, including more intricate compounds used to provide various benefit to plants and bacteria, are also formed. Land plants direct a significant portion of photosynthetic products to their roots, some of which are released to the soil as soluble carbon compounds; products termed as rhizoexudates [14].
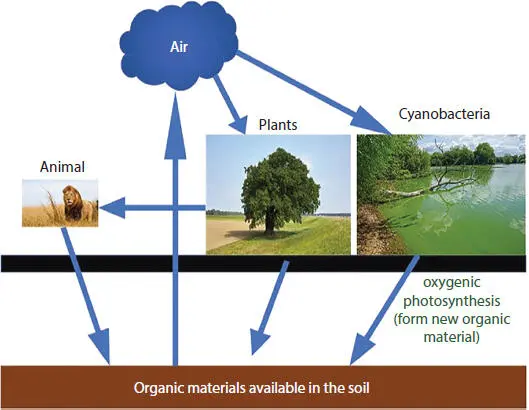
Figure 1.1 Schematic diagram illustrating the biological contributions to the carbon cycle via terrestrial soils.
When plants and bacteria die, their organic constituents are dispersed in soils through decomposition by soil micro-organisms. That decomposition releases much of the CO 2they captured during photosynthesis making its way out of the soil to return to the atmosphere ( Figure 1.1). This organic matter-soil decomposition cycle contributes CO 2output to overall soil respiration that includes respiration of plant root and flora and fauna that live in the soil. In addition to the contributions of plants, algae, and cyanobacteria to the carbon cycle through soils, there is a substantial sub-cycle that is related to contributions from animals. The animals consume CO 2in the form of food, with animal excrements and corpses returning to the soil and being decomposed along with plant, algae, and cyanobacteria remnants.
1.1.1 Soil Decomposition Processes
Animals that live in the soil vary from clearly visible spineless animals such as woodlice, centipedes, and earthworms, to smaller, microscopic-scale animals, the mesofauna, including mites (arthropods), springtails (Collembola a hexapod), and enchytraeidae worm-like (microdrile oligochaete) creatures. Some of the smallest animals in the soil, such as nematodes and protozoa, are among the most effective in the soil decomposition processes [15]. Soil organisms of all sizes and types collectively consume plant, animal, and bacterial debris in the soil. They do this by communition, or the reduction of material from one average particle size to a smaller average particle size, using various physical and biochemical techniques. Fungi and bacteria play a key role in breaking down the structural fabrics of plant materials. These groups are able to convert cellulose and lignin into soluble materials applying complex enzymes to do so. Subsequently, the soluble materials produced are absorbed by the organisms and further metabolized.
Initially, dead plant material located above the ground are, for the most part, decomposed above ground on the soil surface. The soil organisms, weather, and industrial-scale anthropogenic mechanical process such as ploughing, play the substantial role in initiating above-ground and near-surface soil decomposition. In some specific cases, for instance, peat formed in bogs and swamps, the dead plant substances stay at the surface of the soil without time to progress through the complete decomposition process. Instead, it becomes rapidly inhumed by other dead plant substances being added from above, isolating it from abundant oxygen supplies.
A consequence, at completion of the soil decomposition processes, is that carbon is ultimately conveyed from the decaying matter into fungi and soil bacteria. This material is known as microbial biomass. Microbes generate and use this biomass to provide their energy requirements and to create new microbial biomass for growth. That carbon used for energy is converted to CO 2and contribute to soil respiration. However, that portion of the carbon transformed into new microbial biomass, ultimately, is itself consumed or decays upon the demise of the micro-organism and contributes to the ongoing cycle of decomposition. Each successive step in the soil decomposition process involves the consumption of dead biomass by soil organisms, mainly fungi and soil bacteria. Thus, specific carbon molecules pass through many cycles of decay and ultimately end up, over time, either re-emitted to the atmosphere (soil respiration) or fixed by carbon mineralization in the subsurface. On a global scale, the amount of carbon mineralized by soil decomposition is approximately equal to the carbon arriving the soil less the amount re-emitted by soil respiration. Soil respiration returns about 60 Pg a −1(petagram per year; equivalent to 60 gigatonnes) of carbon to the atmosphere, which is about half of the carbon entering the soil [14].
Total carbon accumulating in soils, termed as net primary production (NPP) from organic sources, varies significantly depending on local climatic conditions, vegetation zones, and ecosystems. NPP varies from about 0.5 t C ha −1a −1(tonnes of carbon per hectare per year) in deserts to about 4 t C ha −1a −1in grasslands to about 10 t C ha −1a −1in tropical rain-forests [14]. A direct positive relationship also exists between NPP and the magnitude of carbon released by soil respiration [16].
1.1.2 Organic Compounds Present in Soils
Organic organisms and compounds present in soils are diverse, and the provenance of some of the organic molecules, not present in organisms or identifiable fragments of organisms, is often uncertain. The type and quantity of organic material accumulating in soils is influenced by seasonal weather and biological life cycles. Decomposition processes tend to target the simple biochemical molecules initially; amino acids, nucleic acids, proteins, and sugars. Degradation of the more complex structured molecules, cellulose, hemicellulose, pectins, and polymers takes much longer. Lignins, present in the cell walls of wood and tree bark, takes the longest time to be broken down.
Читать дальше













