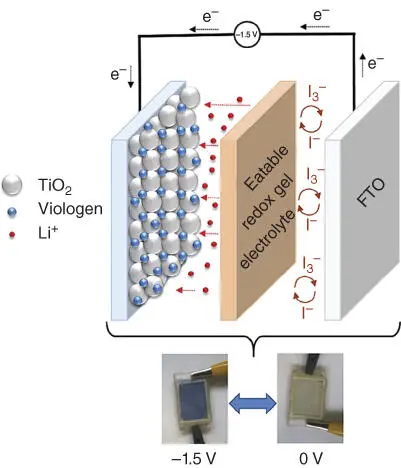
Figure 2.12 An ECD structure containing a gel electrolyte composition combining lithium iodide LiI in 1‐butyl‐3‐methylimidazolium iodide (BMII) ionic liquid, triiodide I 3 −/I −redox mediator, and biodegradable gelatin.
Source: Reproduced with permission of Danine et al. [134].
Moreover, gelatin‐based electrolyte can be used in reflective ECD. Reflective ECD with glass/Cr/PB/electrolyte/CeO 2–TiO 2/ITO/glass configuration was reported to reveal a change from 8% to 15% at 450 nm for bleached and colored states.
Tihan et al. have used a new DNA–LiClO 4‐based solid PE in an ECD ( Figure 2.13) [135]. The novelty of the present research consists in the use of DNA membrane with different LiClO 4ratios in order to achieve new EC windows with good performances [135].
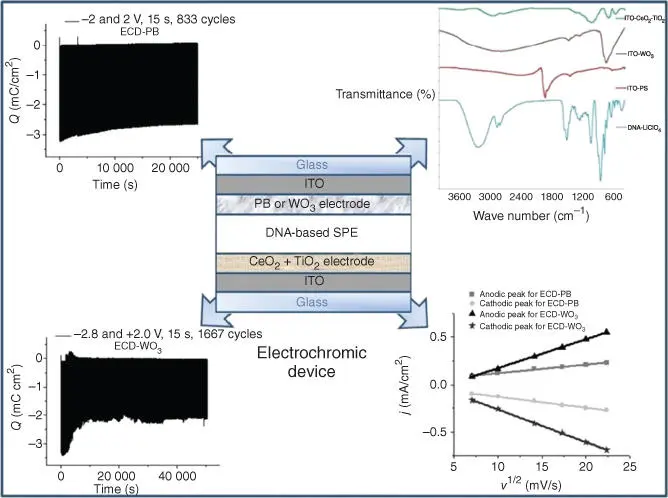
Figure 2.13 An electrochromic device containing DNA‐based electrolyte.
Source: Tihan et al. [135].
2.4 Conclusion and Future Outlook
The field of PE is steadily broadening, as more and more various application are presented. Although lithium‐ion‐based electrolytes have been commercialized, such as applied in pull down window shades on Boeing 787 Dreamliner, smart glass in Ferrari 575 M Superamerica, and Flexity 2 light rail vehicles, some disadvantages such as poor safety, easy leakage, and unstable electrochemical performance limit its further development and wider applications. In this chapter, we have provided fundamental understanding of requirements for EC applications. In the meantime, recent progresses on polymer‐based electrolytes were summarized, including PEO/PVDF/PMMA gel polymer, self‐healing polymer, cross‐linking polymer, ceramic polymer, IL polymer, and gelatin PEs. The use of composite PEs has been identified as a promising method to improve the performance of electrolytes.
Table 2.1 Polymer hosts generally studied with the examples of gel polymer electrolyte complexes and their respective highest ionic conductivities.
| Polymer electrolyte |
EC materials |
Ionic conductivity (S/cm) |
Electrochemical stability window (V) |
Optical modulation (%) |
Stability |
References |
| p (trimethylenecarbonate [TMC])/PEO |
Prussian Blue/PEDOT |
1.33 × 10 −6 |
−1.5 ∼ 1 |
8 ∼ 30 |
Full color switch (>600 s) |
[138] |
| PEO/PVDF |
TiO 2 |
6.98 × 10 −6 |
— |
90 |
— |
[15] |
| PEO/LiClO 4 |
PEDOT:PSS |
4.2 × 10 −4 |
−3.0 ∼ 3.0 |
22 |
No significant change |
[139] |
| P(VDF‐TrFE)/PEO |
PEDOT:PSS |
6.79 × 10 −4 |
−1.0 ∼ 0.5 |
60 |
— |
[140] |
| PEMA/poly (vinylidene fluoride) (PVdF)‐HFP |
WO 3//CeO 2–TiO 2 |
1.46 × 10 −6 |
−1.3 ∼ −0.8 |
81 |
— |
[141] |
| PVdF‐HFP/silane‐functionalized ZrO 2 |
PANI:DBSA |
1.78 × 10 −3 |
−4.0 ∼ 4.0 |
70 |
— |
[142] |
| PVDF‐HFP/POEI‐ISF 4 |
Nanofibers |
8.62 × 10 −3 |
0 ∼ 1.2 |
68.7 |
95.5% of Δ T |
[143] |
| PVDF‐ co ‐HFP/[EMI][TFSI] |
1,1′‐bis(3‐fluoro‐4‐(trifluoromethyl)phenyl)‐4,4′‐bipyridinium bis(trifluoromethylsulfonyl)imide (TFMFPhV(TFSI) 2) |
6.7 × 10 −3 |
−0.35 ∼ 0 |
∼73 |
∼14% decrease of Δ T after 24 h |
[144] |
| IL/PVDF‐HFP |
PEDOT:PSS |
1.13 × 10 −3 |
−1.4 ∼ 1.4 |
∼24 |
— |
[54] |
| TMPD/HV(BF 4) 2/SN/PVDF‐HFP |
TMPD/HV(BF 4) 2/succinonitrile |
1 × 10 −3 |
0 ∼ 0.9 |
60.1 |
74.7% of the original Δ T after 2000 cycles |
[145] |
| [Emim]BF 4/PMMA |
WO 3 |
2.9 × 10 −3(RT) |
−3.0 ∼ 1.5 |
88 |
— |
[146] |
| Poly(ether ether ketone) membrane |
IR‐VEDs |
6.8 × 10 −3 |
−1.0 ∼ 1.0 |
47 |
Good cycling stability |
[147] |
| PVDF‐HFP:PMMA/LiClO 4 |
— |
2.83 × 10 −4 |
— |
— |
— |
[148] |
| PMMA/SN‐PC |
WO 3//brain natriuretic peptide (PBNPs) |
1.46 × 10 −3 |
−2.5 ∼ 2.0 |
52.4 |
44.5% after 2250 cycles |
[149] |
| Poly(ε‐caprolactone) (PCL)/SiO 2 |
PEDOT: PSS |
5.2 × 10 −3 |
−4.0 ∼ 1.0 |
30.6 |
Good stability (100 cycles) |
[150] |
| Polyvinyl butyral (PVB)‐based GPEFs |
WO 3//Ni 1−xO |
4.0 × 10 −5 |
−2.0 ∼ 2.0 |
65.8 |
Fading of 4.8% (16 h) |
[151] |
| PAN–DMF–LiTFSI |
WO 3//CeO 2–TiO 2 |
2.54 × 10 −4 |
−2.0 ∼ 1.0 |
29.1 |
— |
[152] |
| PADA |
LiNiO//WO 3 |
1.33 × 10 −2(RT) |
−2.30 ∼ 2.30 |
61 |
58.4% after 50 cycles |
[153] |
| PVDF based |
P(PVK‐ co ‐EDOT) |
— |
−0.9 ∼ 0.5 |
39.1 |
— |
[154] |
| 2‐APPG /PMDA/ICS |
WO 3film |
1.01 × 10 −3 |
−3.5 ∼ 2.5 |
38.2 |
450 cycles |
[155] |
Table 2.2 Examples of self‐healingand cross‐linking polymer electrolyte complexes and their respective highest ionic conductivities.
| Electrolyte |
Ionic conductivity (S/cm) |
Electrochemical window (V) |
Application |
References |
| B‐PVA/KCl/graphene oxide (GO) |
47.5 × 10 −3 |
0 ∼ 1.0 |
Flexible energy‐storage device |
[156] |
| SiO 2–UPy |
8.0 × 10 −5 |
0 ∼ 6.0 |
Lithium‐ion batteries |
[157] |
| P(AA‐VIm‐VSN) |
1.26 × 10 −4 |
−1.2 ∼ 0.3 |
Flexible electrochromic devices |
[158] |
| [P(PO/EM)]/lithium trifluoro(perfluoro‐tert‐butyloxyl)borate (LiTFPFB) |
1.55 × 10 −4(70 °C) |
3 ∼ 4.2 |
Lithium metal batteries |
[159] |
| Zn–metal‐organic gel (MOG) |
— |
0 ∼ 2.1 |
Flexible supercapacitor |
[160] |
| Poly(ethylene‐co‐acrylic lithium (fluoro sulfonyl)imide) (PEALiFSI) |
5.84 × 10 −4 |
2.5 ∼ 4.0 |
Solid‐state Li‐ion batteries |
[161] |
| Li + ‐PEO |
— |
−4 ∼ 4 |
Micro‐supercapacitors |
[162] |
Although extensive researches have been devoted to the polymer‐based electrolytes, some basic issues still need to be addressed before commercialization. Above all, ionic conductivity is still on lower orders of magnitude than lithium‐based liquid electrolytes. Temperature is one of the most dominant parameters for determining the ionic conductivity. In particular, ionic conductivities in polymer‐based solid electrolytes drop dramatically at lower temperatures. Moreover, the mechanism of interfacial interaction between electrolyte and an EC layer is still unknown.
For now, the GPEs, typically based on PEO/PVDF/PMMA, have also been extensively explored, but they exhibit too low ionic conductivity at room temperature (≈10 −5S/cm) and poor resistance to oxidation at relatively high voltage. Poor mechanical performance limits the IL electrolyte from increasing the ionic conductivity. Safety issues cannot be neglected. Developing a self‐healing electrolyte that simultaneously exhibits excellent EC properties and good self‐healing behavior at low temperature is still an unmet challenge. The cross‐linked composite PE could effectively encapsulate the electrolyte solution without solvent leakage and exhibit favorable interfacial characteristics. However, low ionic conductivity at room temperature still need to be improved. The difficulty of ceramic PEs is how to construct good dispersion and enhance the interaction between filler and polymer, which restricts the further improvement of ionic conductivity. Gelatin‐based PEs have proved to be one of the alternative binders, but its rheological behavior in the tested conditions inhibited such material to be used at industry level.
Читать дальше














