2.3.1.3 PVDF‐Based Polymer Electrolytes
PVDF is another popular host material for electrolytes and has recently been widely used in ECDs. PVDF offers many advantages, such as good thermomechanical properties, fairly high permittivity, high hydrophobicity, thermal and chemical stabilities, and chemical resistance. As a semi‐crystalline polymer, the PVDF crystalline phase provides thermal stability, while the amorphous phase provides the flexibility required for ECDs. PVDF can be soluble in high boiling point and commercial solvents, such as N ‐methyl‐2‐pyrrolidone (NMP) and dimethylformamide (DMF). However, comparing with lithium salt, PVDF, which is expected to have a low donor number (DN), is insoluble and cannot be used effectively in polymer salt complexes. For EC application, PVDF‐based PEs have been identified as interesting candidates and their study is in progress. As was reported by Fabrettos group, an ECD was prepared by using PVDF for studying the coloration efficiency [53]. P(VDF‐TrFE) as GPE was also studied as potential PE in ECD with polyaniline as EC materials [1]. The gel electrolyte device reached an average ionic conductivity of 2.84 × 10 −5S/cm and shows stable and reversible light modulation up to 65% in gel state. It was found that the gel‐state device was affected by the number of free ions, while the movement of ions in the electrolyte bulk and the modulation of light in the semisolid device are indicated by the electrolyte/EC material interface.
The ionic conductivity of PVDF PEs can be enhanced by incorporating substantial amounts of plasticizers or combining with IL. Jia et al. have studied 1‐butyl‐3‐methylimidazolium hexafluorophosphate‐loaded SCCO 2‐treated electrospun P(VDF‐HFP) membrane as an electrolyte in EC device [54].
Recently, Reynold's group reported paper‐based ECDs consisting of PEDOT:PSS electrodes and [EMI][TFSI]/PVDF‐HFP ion gel electrolyte layer (Figures 2.2and 2.3) [55]. The ECDs incorporating an ion gel electrolyte were demonstrated where a magenta‐to‐colorless device achieves a color contrast (Δ E* ) of 56, attributing to a highly color‐neutral bleached state of the extracellular protein (ECP) ( a* = −0.5, b* = 2.9). It was found that the gel‐state device was affected by the number of free ions, while the movement of ions in the electrolyte bulk and the modulation of light in the semisolid device were indicated by the electrolyte/EC material interface.
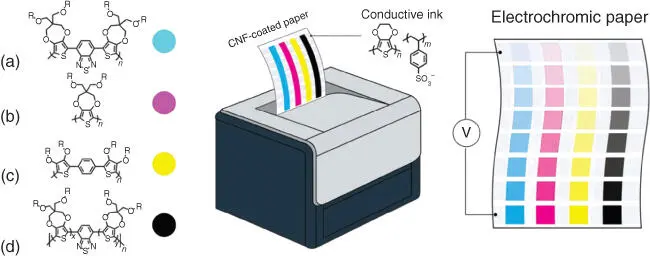
Figure 2.2 Schematic showing printing of colored‐to‐clear electrochromic paper incorporating cellulose nanofiber (CNF)‐coated paper substrates and PEDOT:PSS electrodes as well as the repeat‐unit structures of (a) ECP‐Cyan, (b) ECP‐Magenta, (c) ECP‐Yellow, and (d) ECP‐Black. R = ethylhexyl.
Source: Lang et al. [55].
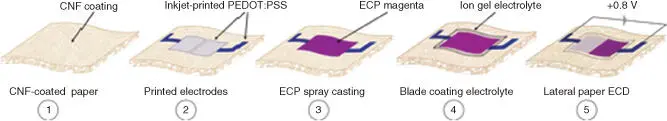
Figure 2.3 A fabrication process for lateral paper ECDs showing inkjet‐printed PEDOT:PSS electrodes, deposition of ECPs, and [EMI][TFSI]/PVDF‐HFP ion gel electrolyte layer. Devices are operated by applying a 0.8 V bias across the two lateral pixels.
Source: Lang et al. [55].
2.3.2 Self‐Healing Polymer Electrolytes
A self‐healing material is a material that possesses abilities to partially repair damage and restore mechanical properties during its service lifetime [56]. Self‐healing materials are particularly attractive in the field of smart materials because of their inherent ability to repair cracks, thereby avoiding such risks, reducing waste, and improving equipment life, durability and reliability. It is believed that the use of an EC polymer film with self‐healing properties would be an effective approach to overcome the risk of the scar generation in ECDs. Until now, self‐healing polymers based on the Diels–Alder (DA) reaction [57] is an effective method for the implementation of intrinsic self‐healing into functional materials, which have been explored for various electronic and optoelectronic applications like conducting films [58], organic light‐emitting devices [OLEDs] [59], supercapacitors, etc. [60] Self‐healing can come up from the microscopic to macroscopic level. Some properties in engineering materials may degrade over long time because of environmental conditions, fatigue or operation damage. Self‐healing materials can address this kind of degradation. The ability to self‐heal upon breakdown or damage depends on various kinds of physical interactions such as hydrogen bonding [61], π–π stacking [62], ion dipole interaction [63], or chemical interactions such as disulfide bond [64, 65] and imine bond. [66] They heal autonomously or in response to a multitude of externally applied stimuli such as pH change, light [67], electricity, temperature [68], and pressure. Geubelle and coworkers first reported a completely autonomous man‐made self‐healing material [69]. Later, an epoxy system containing microcapsules covered with (liquid thermosetting) monomers was explored [70]. If a microcrack occurs in the system, the microcapsule breaks and the crack will be filled by the monomer. This novel self‐healing system could be performed well in polymers and polymer coatings [71].
Zhang and coworkers [72] prepared a dual physically cross‐linked hydrogel containing the hydrogen bonding between poly(acrylamide‐ co ‐acrylic acid) and PVA, hence producing self‐healing abilities in hydrogel. As was reported, ion gels with self‐healing performance, which composed of polymer networks and electrolyte solutions, have attracted great attention [73]. Different strategies and approaches to devise self‐healing materials in ECs have been investigated. For example, multifunctional EC ion gels were enabled by a blend of active methyl viologen dication in polystyrene‐block‐poly(methyl methacrylate)‐blockpolystyrene (PS‐PMMA‐PS) triblock copolymer with ILs [74]. Poly[styrene‐ran‐1‐(4‐vinylbenzyl)‐3‐methylimidazolium hexafluorophosphate] (P[S‐r‐VBMI][PF6]) random copolymer ( Figure 2.4) [75], poly(styrene‐ran‐methyl methacrylate) (PS‐rPMMA) random copolymer [76], (poly(methyl methacrylate)‐ b ‐polystyrene) 6star‐shaped block copolymer ( Figure 2.5) [77], and poly(vinylidene fluoride‐ co ‐hexafluoropropylene) (PVDF‐ co ‐HFP) [78], etc. matrices have been developed for achieving self‐healing solid PEs. The ion conductivity of PVDF‐ co ‐HFP‐based polyelectrolytes could be improved by using various PVDF‐ co ‐HFP‐based polymers and amorphous polymer electrolytes such as PEG [78], PMMA [79] and poly(vinyl acetate) (PVAC) [80, 81]. In addition, materials containing IL/polymer blends for self‐healable EC have been reported in Leong's group [82]. The interaction of polymer blends or the inclusion of reaction sites and addition of ILs may induce rapid healing at lower temperatures, attributing to the increased fluidity and inhibition of the glass transition temperatures. Moreover, self‐healing polymers based on DA reaction [83] is another effective method for self‐healing functional materials. A polymer N ‐(4‐aminophenyl)‐ N ‐phenylbenzene‐1,4‐diamine maleimide (DATPFMA) was the first report on a bifunctional self‐healing EC polymer, which exhibited superior cyclic stability and colors variations [57]. The N , N′ ‐bis(4‐aminophenyl)‐ N , N 0‐diphenyl‐1,4‐phenylene diamine used as core and electro‐active unit for synthesizing the polymer poly(pentafluorophenyl methacrylate) (PPFMA) provided more electrochemical active sites to enhance the EC performance ( Figure 2.6) [84]. This DA polymer can also repair cracks by retro‐DA and DA reaction for the self‐repair capability [57].
Читать дальше














