The main cell types of the pancreatic islets are β‐cells that produce insulin, α‐cells that secrete glucagon, δ‐cells that produce somatostatin and PP‐cells that produce pancreatic polypeptide. Different islet cell types can be identified by various immunostaining techniques. β‐cells are the most numerous cell type and are located mainly in the core of the islet, while α and δ cells are located in the periphery ( Figure 5.2).
Islet cells interact with each other through direct physical contact and through paracrine effects of their hormone products (e.g. glucagon stimulates insulin secretion and somatostatin inhibits insulin and glucagon secretion) ( Figure 5.3). The blood flow within the islets is organised centrifugally so that the different cell types are supplied in the sequence β → α → δ. Insulin also has an ‘autocrine’ (self‐regulating) effect that alters the transcription of insulin and glucokinase genes in the β cell.
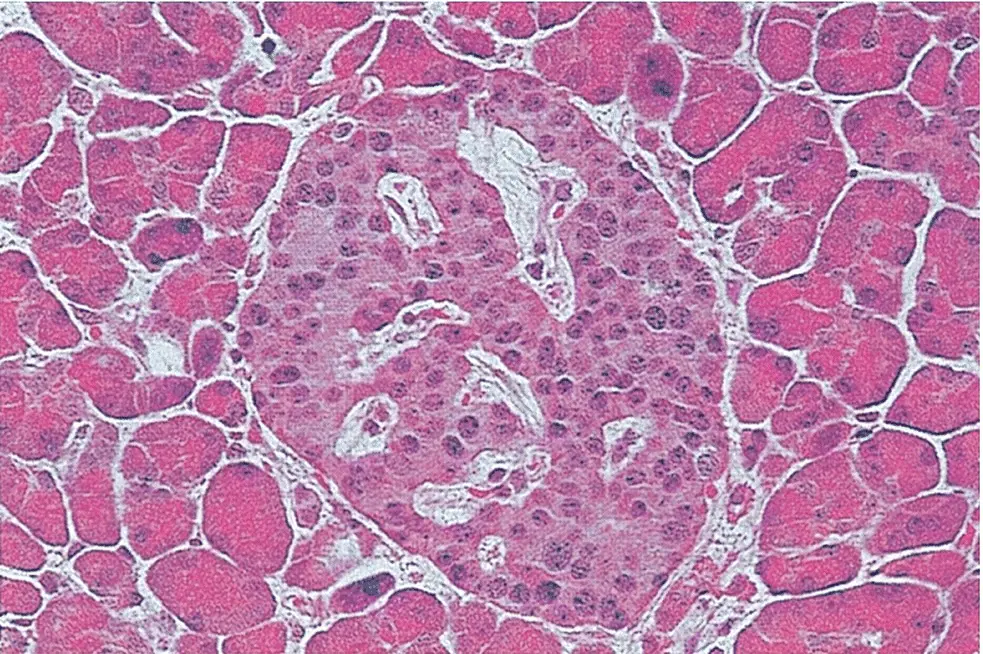
Figure 5.1 A section of normal pancreas stained with haematoxylin and eosin. As observed by Paul Langerhans, the islet in the centre is identified easily by its distinct morphology and lighter staining than that of the surrounding exocrine tissue (original magnification ×350).

Figure 5.2 The localisation of pancreatic hormones in human islets. (a) Insulin immunostained in the majority of cells that form the core of the islet (peroxidase–antiperoxidase immunostain with haematoxylin counterstain). (b) Insulin mRNA localised by in situ hybridization with a digoxigenin‐labelled sequence of rat insulin cRNA (which cross‐reacts fully with human insulin mRNA). (c) Peripherally located α cells immunostained with antibodies to pancreatic glucagon using the same method as for (a). (d) Weakly immunoreactive PP cells in the epithelium of a duct in the ventral portion of the pancreatic head. Magnifications approximately ×150.
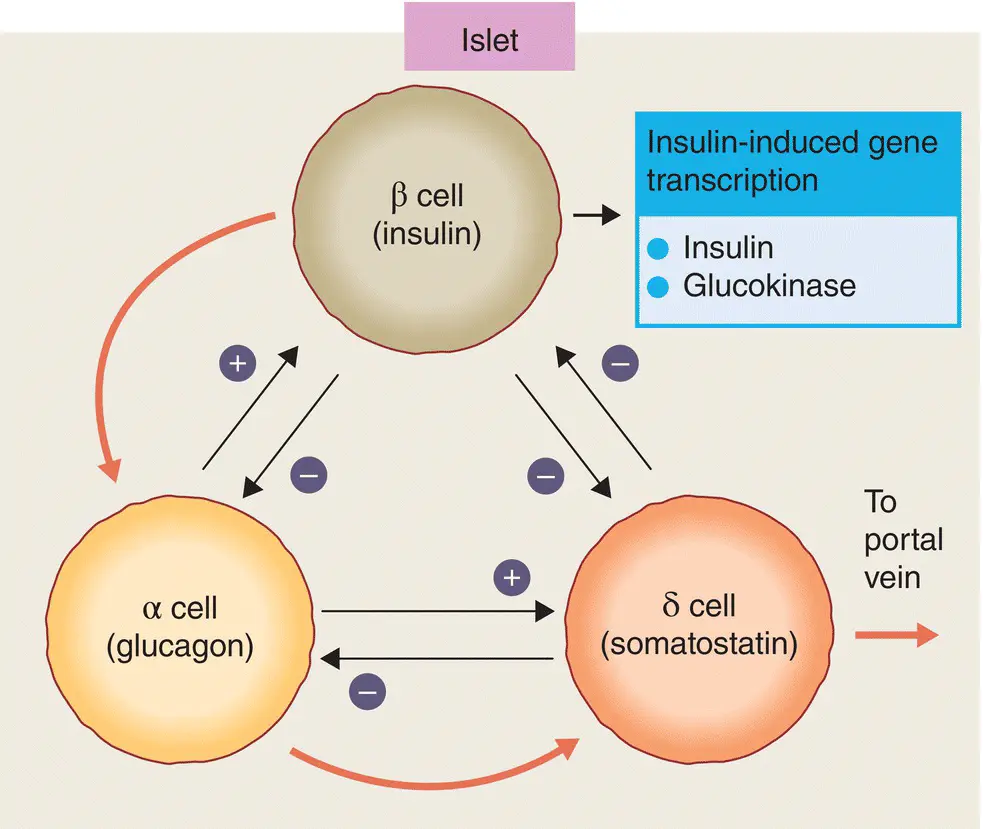
Figure 5.3 Potential interactions between the secretory products of the major islet cell types. Black arrows indicate paracrine stimulation or inhibition. The direction of blood flow within the islet is indicated by the red arrows.
Pancreatic islets are densely innervated with autonomic and peptidergic nerve fibres ( Figure 5.4). Parasympathetic innervation from the vagus stimulates insulin release, while adrenergic sympathetic nerves inhibit insulin and stimulate glucagon secretion. Other nerve fibres that supply the pancreas release peptides which also regulate pancreatic function, e.g vasoactive intestinal peptide (VIP) stimulates the release of all islet hormones and neuropeptide Y (NPY) inhibits insulin secretion. Neural pathways are important in the regulation of pancreatic function.
Pancreatic β‐cells may change in size, number, and function during normal aging and development ( Figure 5.5). β cell mass is determined by the net effect of four independent mechanisms: (i) β cell replication (i.e. division of existing β cells), (ii) β cell size, (iii) β cell neogenesis (i.e. emergence of new β cells from pancreatic ductal epithelial cells), and (iv) β cell apoptosis. The contribution made by each of these processes is variable and may change at different stages of life.

Figure 5.4 Structure of a pancreatic islet, showing the anatomical relationships between the four major endocrine cell types. NPY, neuropeptide Y; VIP, vasoactive intestinal polypeptide.
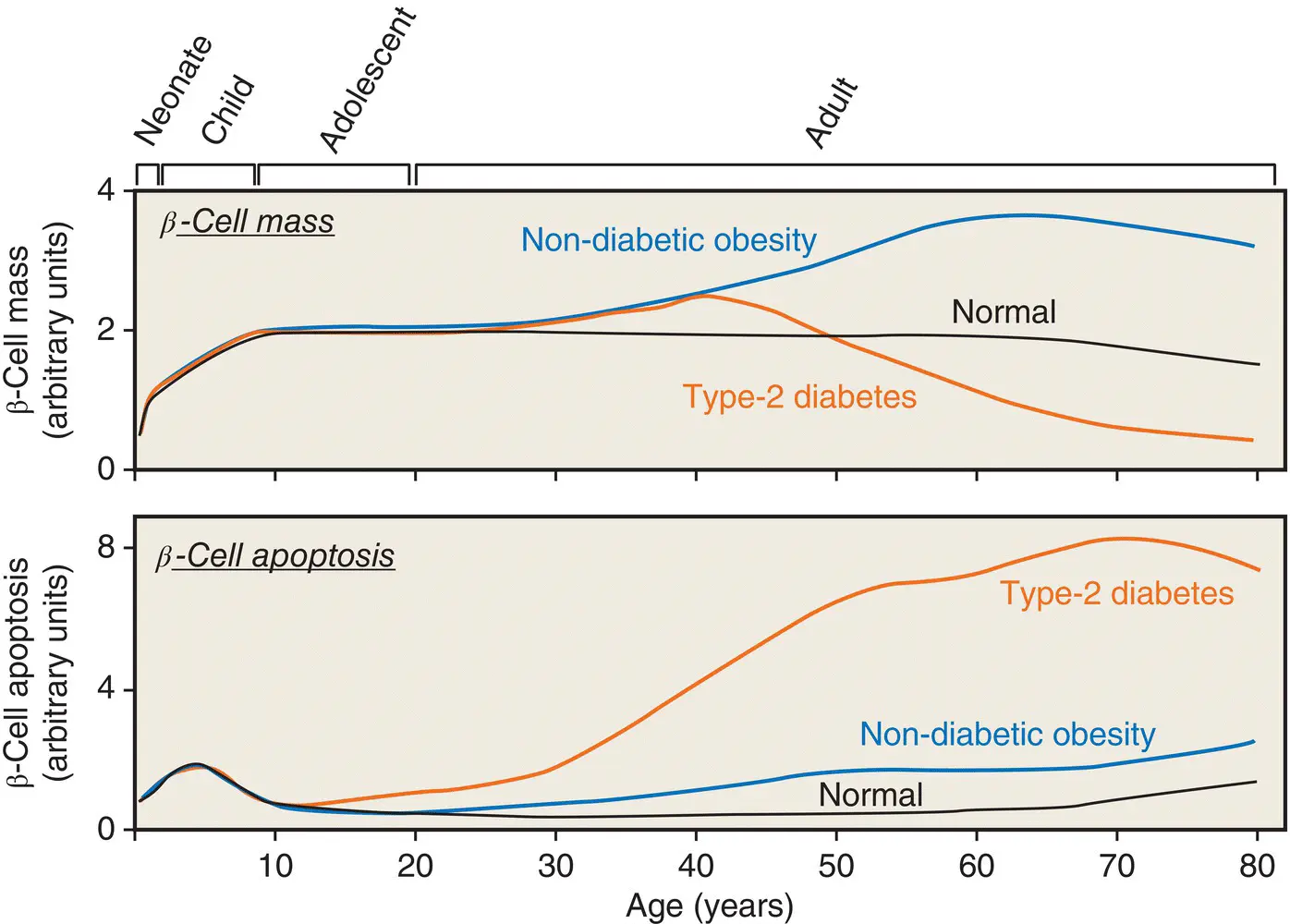
Figure 5.5 A hypothetical model for postnatal pancreatic β cell growth in humans.
Adapted from Rhodes et al. Science 2005; 307: 380–384.
Insulin synthesis and insulin polypeptide structure
Insulin consists of two polypeptide chains linked by disulphide bridges; the A‐chain contains 21 amino acids and the B‐chain contains 30 amino acids ( Figure 5.6). In the circulation, insulin exists as a monomer of 6000 Da molecular weight. The tertiary (three‐dimensional) structure of monomeric insulin consists of a hydrophobic core buried beneath a surface that is hydrophilic, except for two non‐polar regions involved in the aggregation of the monomers into dimers and hexamers.
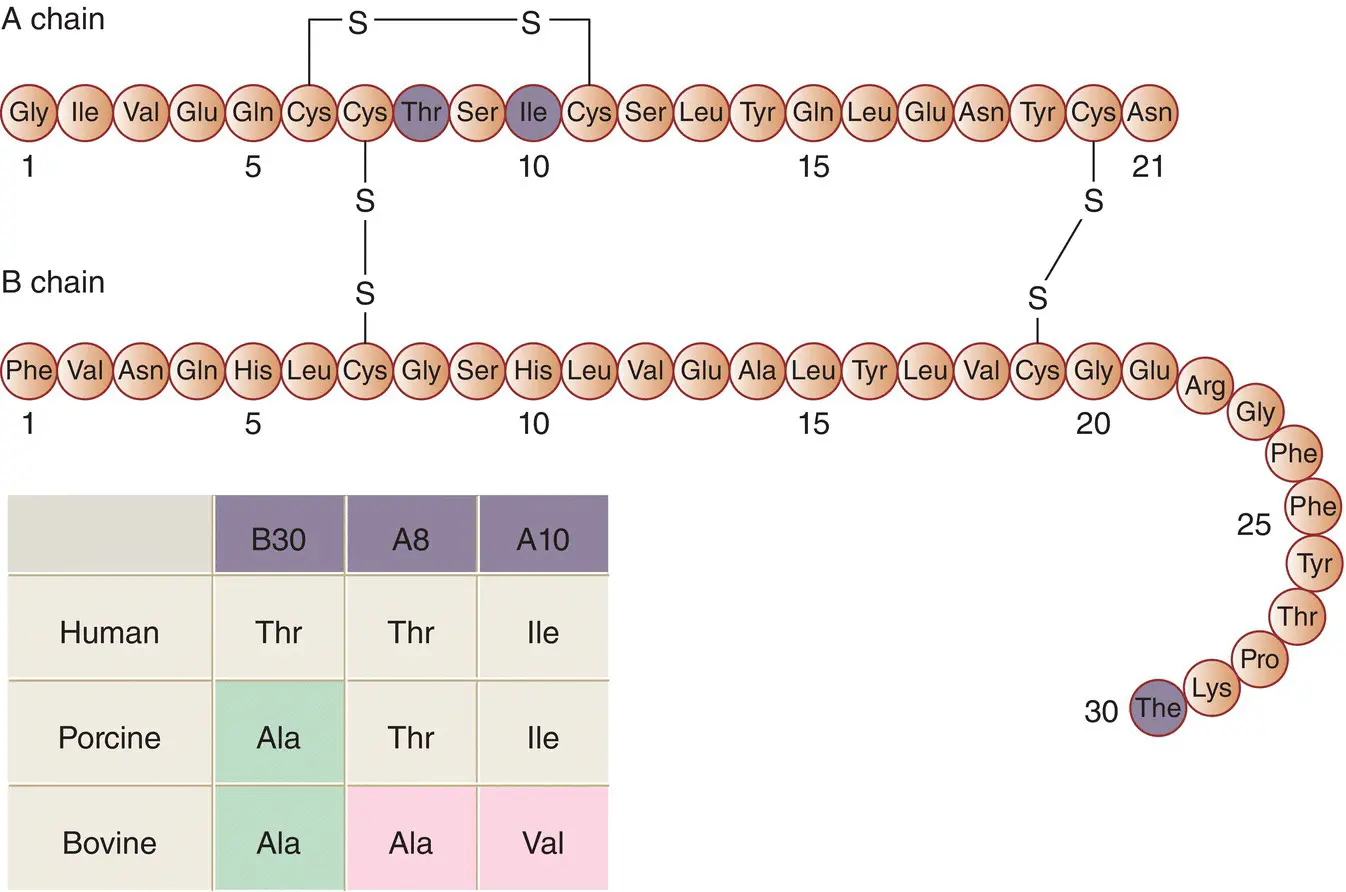
Figure 5.6 The primary structure (amino acid sequence) of human insulin. The highlighted residues are those that differ in porcine and bovine insulins, as shown in the inset.
In concentrated solution (such as in the insulin vial supplied by the pharmaceutical company for injection) and in crystals (such as in the insulin secretory granule), six monomers self‐associate with two zinc ions to form a hexamer ( Figure 5.7). This is of therapeutic importance because the slow absorption of native insulin from the subcutaneous tissue partly results from the time taken for the hexameric insulin to dissociate into the smaller, more easily absorbed monomeric form.
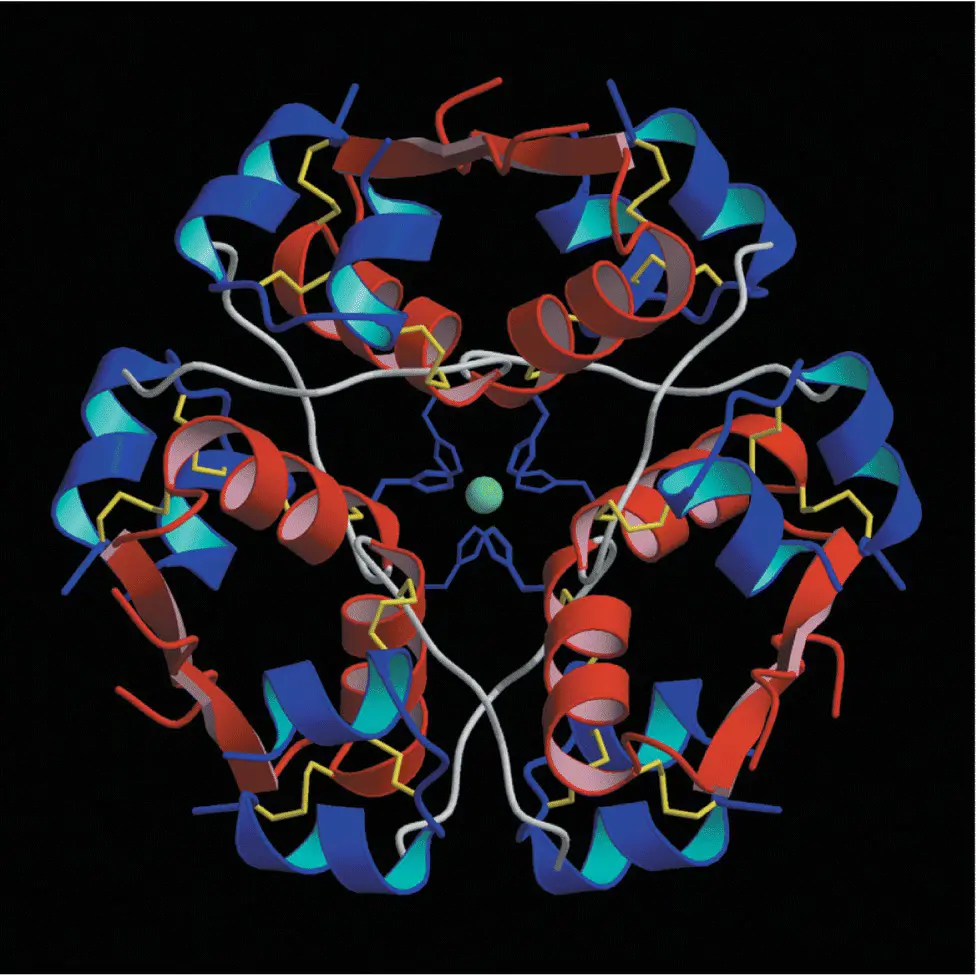
Figure 5.7 The double zinc insulin hexamer composed of three insulin dimers in a threefold symmetrical pattern.
Insulin is synthesised in the β cells from a single amino acid precursor called proinsulin ( Figure 5.8). Synthesis begins with the formation of an even larger precursor, preproinsulin, which is cleaved by protease activity to proinsulin. The gene for preproinsulin (and therefore the ‘gene for insulin’) is located on chromosome 11. Proinsulin is packaged into vesicles in the Golgi apparatus of the β cell; in the maturing secretory granules that bud off it, proinsulin is converted by enzymes into insulin and connecting peptide (C‐peptide).
Insulin and C‐peptide are released from the β cell when the secretory granules are transported (‘translocated’) to the cell surface and fuse with the plasma membrane (exocytosis) ( Figure 5.9). Microtubules, formed of polymerised tubulin, probably provide the mechanical framework for granule transport, and microfilaments of actin, interacting with myosin and other motor proteins such as kinesin, may provide the mechanical force that propels the granules along the tubules. Although the actin cytoskeleton is a key mediator of biphasic insulin release, cyclic GTPases are involved in F‐actin reorganization in the islet β cell and play a crucial role in stimulus‐secretion coupling.
The ‘regulated pathway’, with almost complete cleavage of proinsulin to insulin, normally accounts for about 95% of the β cell insulin production. In certain conditions, however, e.g insulinoma and type 2 diabetes, an alternative ‘constitutive’ pathway operates, in which large amounts of unprocessed proinsulin and intermediate insulin precursors (‘split proinsulins’) are released directly from vesicles that originate in the endoplasmic reticulum ( Figure 5.10).
Читать дальше



















