Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Acid‐catalyzed depolymerization of cellulose in ILs opens numerous avenues to generate significantly value‐added chemicals from low‐molecular‐weight sugars. Pertinent examples include the fermentative production of ethanol for biofuel applications, or transition metal‐catalyzed synthesis of hexitols for food and medical uses [ 34, 69, 70]. Another interesting application is found in their transformation into alkyl glycosides, a class of biodegradable surfactants with widespread employment in differentiated products such as cosmetics, body care, and cleaning formulations [71]. The Corma research group from the Polytechnic University of Valencia published a series of papers dedicated to the synthesis of alkyl glycosides through the hydrolysis of cellulose into low‐molecular‐weight carbohydrates in an ionic solvent, followed by glycosidation into alkyl glycosides and alkyl polyglycosides [ 72– 74]. This was accomplished by the addition of long‐chain alcohols, such as 1‐octanol, 1‐decanol, or 1‐dodecanol, after depolymerization of cellulose in [C 4mim]Cl in the presence of acidic resin Amberlyst® 15. The method permitted the production of surfactants in high yield (up to 82 mol%), under mild processing conditions (temperatures around 100 °C) [72]. It is worth noting that alkyl glucosides are commercially synthesized from low‐molecular‐weight carbohydrates or structural polysaccharides in two steps, namely, glycosidation of the substrate with low‐molecular‐weight alcohols, followed by transacetalization with long‐chain alcohols [71]. The production of alkyl glycosides from cellulosic biomass in ILs is therefore a promising alternative pathway to generate bio‐based surfactants. The ongoing challenge relates to the separation of glycosides from ILs and is currently based on conventional chromatographic methods, which are difficult to sustain at larger scale, especially with mid‐priced performance chemicals [74]. Nevertheless, the problem may be potentially solved by the use of simulated moving bed chromatography that has already been applied to the simultaneous recovery of monosaccharides and ionic solvents [C 4mim]Cl at the multigram scale [70]. Simulated moving bed chromatography is largely employed in the fractionation of carbohydrates, and this method demonstrates significant commercial potential [70].
Among the various biorefinery processes, there is a particular focus on the transformation of cellulosic saccharides into furan derivatives [ 4, 75]. These are useful targets because HMF (a cellulose‐derived product) and FF (a hemicellulose‐derived product) are raw materials for the production of biofuels, bioplastics, food additives, and pharmaceuticals [ 75, 76]. The synthesis of furans is well investigated in aqueous media, mostly based on the acid‐catalyzed transformation of low‐molecular‐weight sugars such as fructose, sucrose, and xylose [ 75, 76]. The direct conversion of undervalued polysaccharides into furans in aqueous solvents is difficult, mostly as a consequence of the chemical reactivity of aldehydes in the acidic reaction media [4]. As a case in point, furaldehydes are convertible into LevA and its derivatives ( Scheme 2.2) and also to high‐molecular‐weight by‐products such as humins (condensation products of saccharides and aldehydes), which have a limited scope of applications [ 75, 77, 78]. Additional complexities arise in the planning and execution of these reactions because the conversion of polysaccharides into furans involves aldose–ketose isomerization promoted by Lewis acids ( Scheme 2.2), whose activity is often compromised in aqueous reaction media [ 4, 7].
The abovementioned issues have been largely alleviated by the employment of ILs, often because of the stabilization of the reactive furanoids and the catalyst in the ionic media [ 4, 61, 79]. A commonly applied strategy is the processing of polysaccharides in imidazolium‐based solvents in the presence of metal chloride catalysts (MCl n, M = metal, n = integer) [ 4, 61]. The solvent–catalyst interaction presumably leads to the formation of acidic catalytic complexes, [C nmim] +[MCl n+1] –in the case of 1‐alkyl‐3‐methylimidazolium chloride solvents ( Scheme 2.5), and these complexes tend to promote the requisite Lewis acid‐catalyzed aldose–ketose isomerization [79]. Brønsted acidity is likely achievable by the hydrolysis of some of these species in the presence of water with concomitant formation of metal aquo complexes and (hydrated) hydrogen cations ( Scheme 2.5), as is commonly observed in aqueous systems [80]. Decomposition of imidazolium salts into N‐heterocyclic carbenes and HCl may also be a source of Brønsted acid activity [81]. However, in many instances, the processing requires the addition of protic acids to the reaction media [ 4, 61]. The direct conversion of native cellulose and lignocellulose has been conducted in a cosolvent system comprising [C 2mim]Cl (20–80 wt%, based on the reaction system) and dimethylacetamide (DMA)/LiCl mixture (LiCl, 10 wt%) [50]. Mixtures of DMA and LiCl (present in differing ratios depending on the targeted application) are ionic media that have already been extensively employed in cellulose refining technologies [58]. This solvent system enabled the direct processing of biomass into HMF in good yields (up to 54 mol%, based on the cellulose content, Table 2.1) in the presence of the combined acid catalyst chromium(II) or chromium(III) chloride and hydrochloric acid [50]. With lignocellulosic substrate (corn stover), FF was also obtained (in addition to HMF), owing to acid‐catalyzed reactions of xylans (yield up to 37 mol%, based on the xylan content, Table 2.1). The yields of HMF could be improved during the direct processing of MCC in [C 2mim]Cl in the presence of Lewis acid‐assisted Lewis acid catalyst CuCl 2/CrCl 2(58%) [51], or in the cosolvent system [C 2mim]OAc (1‐ethyl‐3‐methylimidazolium acetate)/1‐(4‐sulfobutyl)‐3‐methylimidazolium methanesulfonate ([C 4SO 3Hmim]CH 3SO 3) in the presence of CuCl 2(70 wt%) [52], but these methods have not been applied to the valorization of native biomass. Combined treatment of lignocellulosic biomass (wood chips and rice straw) in aqueous sodium hydroxide solution (3%), followed by the CrCl 3‐catalyzed transformation in [C 4mim]Cl, enabled inspiring yields of HMF (up to 79 mol%) under relatively mild processing conditions (120 °C, two hours, Table 2.1) [53]. Apparently, treatment in aqueous basic solution removes a substantial portion of lignin and hemicellulose from the biomass, facilitating to the rapid dissolution and hydrolytic processing of the substrate in the ionic solvent. This method may become industrially viable, once the efficient recovery of the reaction system and the targeted furaldehyde have been engineered.
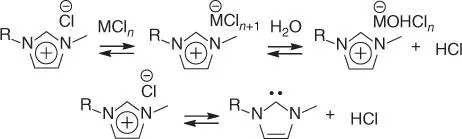
Scheme 2.5 Proposed formation of catalytic species in [C nmim]Cl. R, alkyl; n, integer.
Although ILs are excellent media for the valorization of carbohydrates, these systems suffer some drawbacks, mostly related to the high cost of common ionic solvents, and sometimes to intricacies relating to their recycling [4]. These downsides pose a barrier to their widespread industrial acceptance. In this regard, many researchers are currently investigating less‐expensive ionic solvents for the valorization of biomass [4]. Zinc chloride hydrate solvents, with the conventional formula ZnCl 2· n H 2O (these systems are true ILs with the molecular formula [Zn(OH 2) 6][ZnCl 4] in the case of n = 3), have proved to be suitable for some biorefinery applications [ 35, 54, 82– 86]. Such ionic systems have been historically employed as solvents in cellulose refining technologies and have been found useful in the production of cellulose aerogels, low‐molecular‐weight saccharides, and their derivative sugar alcohols [ 82– 85]. Our recent systematic studies [ 35, 54, 86] demonstrate that ZnCl 2· n H 2O possesses intrinsic catalytic activity, which promotes the conversion of polysaccharides into value‐added molecules ( Scheme 2.2and Scheme 2.6). Moreover, it is possible to adjust the activity of ZnCl 2· n H 2O by manipulating the hydration number n [ 35, 54, 86]. Less‐hydrated media, ZnCl 2·2.5–3.0H 2O, favor the transformation of cellulose into furans, namely, HMF, furyl hydroxymethyl ketone (FHK), and FF ( Figure 2.1). FHK and FF are unusual major dehydration products, and their formation is accordingly seldom recorded for the reactions of cellulose. FHK is considered to originate by the dehydration of the intermediate ketohexose (isomerization product derived from fructose), while FF is thought to form from fructose via an intermediate pentose (e.g. arabinose), as shown in Scheme 2.6. These two furanoids are used as specialty solvents, pharmaceutical intermediates, and in the production of performance resins. Interestingly, the selectivity to these unusual furanoids may be significantly improved when performing reactions in the biphasic system ZnCl 2·3.0H 2O/anisole [ 54, 86]. This biphasic system is especially useful for the production of FF from native biomass because of the simultaneous conversion of both cellulose and hemicellulose into the targeted furaldehyde (yield up to 42 wt% based on cellulose and hemicellulose content in biomass, Table 2.1) [54]. In distinct contrast to less‐hydrated solvents, highly hydrated molten salts ZnCl 2·4.0–4.5H 2O transform cellulose predominantly into HMF (yield up to 21 mol%) and low‐molecular‐weight saccharides (total yield up to 48 wt%, Figure 2.1) [35]. The correlation between selectivity of the products and hydration levels of ILs is presumed to be related to the acidity of the reaction media, which diminishes with rising n , as was shown by pH readings and NMR spectroscopy [35]; however, the exact nature of the catalytic action of ZnCl 2· n H 2O remains to be established. After optimizing the process, high yields of HMF (up to 35 mol%), FF (up to 29 wt%), and sugars (up to 61 wt%) are achievable by performing the conversion of native lignocellulose (corncob and softwood) and algal biomass (macroalga Ulva lactuca or microalga Porphyridium cruentum ) in ZnCl 2·4.25H 2O under relatively mild conditions ( Table 2.1) [35]. In addition, the transformation of lignocellulose in zinc chloride hydrate solvents enabled the recovery of a lignin‐containing residue [ 35, 87]. However, not all types of biomass were found to transform efficiently in the inorganic solvent. For example, native softwood is less amenable for the catalytic conversion ( Table 2.1). Additionally, economical methods to recover products and solvents demand further investigations.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












