Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Cellulose is the most naturally abundant macromolecule on the Earth [36]. Even if this view is contested, lignocellulose is the only large volume biomass to which we have ready access on large industrial scale and where there is a globally distributed large volume industry and supply chain [4]. Cellulose consists of β(1 → 4) linearly linked glucose units and is a principal portion of plant cell walls. Hemicellulose is another polysaccharide present in lignocellulose and is often made of structurally branched xylose units and sometimes other moieties [ 6, 16, 31]. The past few decades have witnessed a significant interest in the acid‐catalyzed processing of cellulosic substances into organic building block chemicals (platform molecules), such as 5‐(hydroxymethyl)furfural (HMF), furfural (FF), levulinic acid (LevA), LacA, and their many derivatives [4].
A key first step in the valorization of polysaccharides is the depolymerization thereof into low‐molecular‐weight sugars, from which other value‐added chemicals are generated ( Scheme 2.2) [ 6, 37]. In their own right, low‐molecular‐weight saccharides are valuable ingredients in the food manufactory and also used as substrates for the fermentative production of ethanol or LacA [ 38– 40]. Polysaccharides are commonly hydrolyzed in aqueous mineral acids in industrial processes [41]. Most technologies are based on thermal hydrolysis of cellulosic biomass in dilute aqueous sulfuric acid solution in batch or percolation reactors, with a typical glucose yield of 55–70% [41]. The glucose yield may be improved to 85%, when conducting the hydrolytic processing of biomass in a countercurrent shrinking bed reactor system at elevated temperatures (up to 225 °C) [42]. This technology has been engineered at National Renewable Energy Laboratory (NREL) by designing a cascade of percolation reactors simulating countercurrent. The available information suggests that this specific process has been demonstrated to bench scale at present [42]. The sustainability of such methods at industrial scale may be compromised by the need for forcing processing parameters [ 40, 43]. On the other hand, complexities arise because of the formation of large amounts of acidic wastewater and solid waste (typically, calcium sulfate after neutralization of sulfuric acid) and also because of the requirement for corrosion‐resistant manufacturing equipment [ 4, 40]. These ongoing challenges have led to the exploration of new efficient methods for the hydrolytic processing of polysaccharides into low‐molecular‐weight derivatives, where high yields and selectivities, accompanied by low levels of waste production, are key parameters.
Solid acid‐catalyzed reactions of polysaccharides in aqueous media have drawn particular interest [40]. The use of solid catalysts is attractive because of the ease of their recovery and sometimes recyclability. An interesting strategy has been devised using a cellulase mimetic catalyst [44], containing both cellulose binding and cellulose hydrolyzing sites, similar to cellulase enzymes. The catalyst is a sulfonated chloromethyl polystyrene resin (CP‐SO 3H) bearing chloride groups (–Cl) with which saccharides and sulfonic groups (–SO 3H) are coordinated to generate the Brønsted acidity requisite for the hydrolysis of the glycosidic linkages ( Scheme 2.3) [44]. The catalyst CP‐SO 3H demonstrated impeccable activity during the processing of microcrystalline cellulose (MCC), or starch (a mixture of α(1 → 4) linearly linked glucose polymer amylose and branched α‐glucose polymer amylopectin), in high yields of glucose (up to 100%). CP‐SO 3H is significantly more efficient than a diluted solution of sulfuric acid under identical processing conditions, or many other reported solid acid catalysts, such as acidic resins, zeolites, carbonaceous acids, or functionalized silicas [ 44, 45]. This success has spread the adoption of cellulase mimetic catalysts based on varied sulfonated chloroorganic materials [46]. Table 2.1provides a summary of the outcomes of several acid‐catalyzed processes.
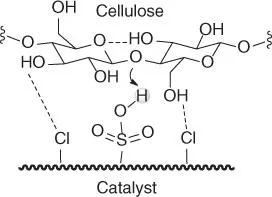
Scheme 2.3 Proposed structure and mechanism of catalytic action of the cellulase mimetic catalyst. Source: Based on Shuai and Pan [44].
The sustainability of solid acids is somewhat undermined by the insolubility of both the cellulose and the catalyst, the latter by design, in aqueous reaction mixtures [ 4, 40]. The heterogeneity of the system limits the number of effective substrate–catalyst interactions during the processing, leading to several technical and economical hurdles. Firstly, there is a need for high loadings of the catalyst [ 4, 44– 46]. This may lead to the production of solid deactivated catalyst waste, if it is not fully recyclable. Another downside relates to the use of pretreated cellulose, instead of largely available lignocellulosic biomass. Pretreatment helps to ameliorate issues pertaining to mass transfer in heterogeneous systems, owing to the reduced physical size and sometimes lower molecular weight of pretreated polysaccharides. Examples of pretreated substrates are MCC, a medium value product obtained by the treatment of wood pulp in diluted aqueous acids, and ball‐milled cellulose [ 16, 58]. Ball milling is arguably the most commonly used method for the pretreatment of cellulosic biomass. Although this approach is efficient at the bench scale and quite effectively depolymerizes cellulose to some extent, it becomes highly energy demanding at the larger scale and remains mostly unemployed in the industry [4]. Some studies, however, claim that some of the significant energy implications associated with this method of pretreatment may be avoided for acid‐assisted mechanochemical depolymerization [ 47, 59]. For instance, mechanochemical depolymerization of beechwood and poplar wood may be realized at kilogram scale by ball milling of biomass in the presence of sulfuric acid [47]. Nevertheless, these processes require subsequent dilution of the pretreated acidified systems with water and hydrolytic treatment under forcing reaction conditions, which does not significantly differ from other processes in aqueous solvents ( Table 2.1). Considering the energy costs accompanied by a need for corrosion‐resistant equipment, such means of depolymerization cannot be accepted as sustainable, unless the energy demands of ball milling can somehow be reduced.
Table 2.1 Conditions and results of the acid‐catalyzed processing of cellulosic biomass into carbohydrates and furansa.
| Substrate | Reaction media | Catalyst | T (°C) | t (h) | Yield glucans (%) | Yield glucose (%) | Yield xylose (%) | Yield HMF (%) | Yield FF (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| MCC | Water | CP‐SO 3H | 120 | 10 | — | 93 | — | — | — | [44] |
| Starch | 2 | — | 100 | — | — | — | ||||
| MCC | H 2SO 4 | — | 1 | — | — | — | ||||
| Ball‐milled MCC | Water | Carbonaceous Cl −, SO 3H‐containing material | 120 | 24 | — | 85 | — | — | — | [46] |
| Ball‐milled MCC | Water | Si 33C 66‐823‐SO 3H | 150 | 24 | 4 | 50 | — | 0 | — | [45] |
| Sulfonated pyrolyzed sucrose | 6 | 27 | — | 1 | — | |||||
| Beechwood b | Water | H 2SO 4 | 145 | 1 | 2 | 76 | 74 | 2 | 6 | [47] |
| Poplar wood b | 2 | 78 | 82 | 2 | 8 | |||||
| Cotton linters | [C 2mim]Cl c | HCl | 105 | 3 d | — | 87 | — | 6 | — | [34] |
| Corn stover | [C 2mim]Cl c | — | 70 | 79 | — | — | ||||
| Sigmacell | [C 4mim]Cl | H 2SO 4 | 100 | 0.75 d | — | 38 | — | — | — | [48] |
| [C 4mim]Cl | HCl | 100 | 0.18 d | — | 21 | — | — | — | ||
| Eucalyptus cellulose | [C 4mim]Cl/ChCl/oxalic c | — | 120 | 10 | 19 | 48 | — | 4 | — | [49] |
| Pinus cellulose | 120 | 10 | 21 | 50 | — | 6 | — | |||
| Corncob | 100 | 2 | 0 | 54 | 35 | 10 | — | |||
| 120 | 4 | |||||||||
| P. cruentum | 100 | 2 | 0 | 55 | 40 | 0 | — | |||
| 120 | 4 | |||||||||
| Cellulose | [C 2mim]Cl/DMA/LiCl | CrCl 2/HCl | 140 | 2 d | — | — | — | 54 | — | [50] |
| Corn stover | CrCl 3/HCl | 47 | 37 | |||||||
| MCC | [C 2mim]Cl c | CuCl 2/CrCl 2 | 120 | 8 | — | — | — | 58 | — | [51] |
| MCC | [C 2mim]OAc/[C 4SO 3Hmim]‐CH 3SO 3 | CuCl 2 | 160 | 3.5 | — | — | — | 70 | — | [52] |
| Wood chipse | [C 4mim]Cl | CrCl 3 | 120 | 2 | — | — | — | 79 | — | [53] |
| Rice straw e | — | — | — | 76 | — | |||||
| Corn husk | ZnCl 2·3.0 H 2O/anisole | — | 120 | 1 d | — | — | — | — | 26 | [54] |
| P. cruentum | — | — | — | — | 42 | |||||
| Corncob | ZnCl 2·4.25 H 2O | HCl | 120 | 1 d | — | 61 | — | 30 | 22 | [35] |
| P. cruentum | — | 49 | — | 35 | 29 | |||||
| Softwood | — | 26 | — | 22 | 11 | |||||
| Ulva lactuca | — | 52 | — | 25 | 15 | |||||
| Inulin | ChCl/oxalic/EtOAc | — | 80 | 2 | — | — | — | 64 | — | [55] |
| Xylans | ChCl/citric/MIBK | AlCl 3 | 140 | 0.58 | — | — | — | — | 69 | [56] |
| P. cruentum | ChCl/oxalic | — | 80 | 2 | 0 | 42 | 73 | 1 | 25 | [57] |
| P. cruentum | ChCl/oxalic/MIBK | — | 100 | 2 | 0 | 68 | 13 | 9 | 44 | |
| 4 | 0 | 32 | 0 | 5 | 72 | |||||
| Softwood | ChCl/oxalic/MIBK | — | 100 | 5 | 0 | 0 | 0 | 1 | 55 |
aWe note that this table show yields of products either in mol% or in wt%; it is recommended to refer to the given references if the accurate evaluation of yields is sought. “—” = not specified; “0” = not detected, or detected in trace amounts; T , reaction temperature; t , reaction time; HMF, 5‐(hydroxymethyl)furfural; FF, furfural; MCC, microcrystalline cellulose; [C 2mim]Cl, 1‐ethyl‐3‐methylimidazolium chloride; [C 2mim]OAc, 1‐ethyl‐3‐methylimidazolium acetate; [C 4mim]Cl, 1‐butyl‐3‐methylimidazolium chloride; [C 4SO 3Hmim]CH 3SO 3, 1‐(4‐sulfobutyl)‐3‐methylimidazolium methanesulfonate; ChCl, choline chloride; DMA, dimethylacetamide; EtOAc, ethyl acetate; and MIBK, methyl isobutyl ketone.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












