Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Conversely, Lewis acid‐catalyzed reactions are those in which the electron‐deficient center of a Lewis acid coordinates to a Lewis basic site on the substrate. The site and strength of the bonding may be predicated based on Pearson's HSAB (hard and soft [Lewis] acids and bases) principles, which predict that soft Lewis acids interact preferentially with soft Lewis bases and hard with hard [19]. When it comes to biomass, the majority of the active sites are hard Lewis bases, and so it is most common to encounter the application of hard Lewis acids to valorization chemistry.
In a third type of acid catalyst system, the mixtures of two different acid catalysts produce synergistic effects to deliver enhanced catalytic activity (Brønsted or Lewis) via an assisted acidity mechanism [ 20, 21]. The assisted acid systems can be constructed from mixtures of two Brønsted acids (Brønsted acid‐assisted Brønsted acid catalysts), two Lewis acids (Lewis acid‐assisted Lewis acid catalysts), or Brønsted and Lewis acids (Brønsted acid‐assisted Lewis acid catalysts or Lewis acid‐assisted Brønsted acid catalysts) [20]. Under specific types of conditions, these mixtures can turn into superacidic systems [22], imparting very significant catalyst activity. A pictorial manifestation of the activation of the substrate by different types of acid catalysts is represented in Scheme 2.1, and throughout this chapter, we will make reference to the different types of acidity that are, or may be, at play.
As is evident from Scheme 2.2, acids can promote the valorization of all main classes of biomacromolecules. Only a few existing acid‐catalyzed processes can be considered to be sustainable, usually because of the nature of the substrates employed. Most methods that show promise in the laboratory are based on transformations of refined edible substances, such as low‐molecular‐weight saccharides and oligosaccharides, vegetable oils, or refined proteins [ 1, 2, 4, 23]. The use of food products for the production of chemicals fails to meet sustainability requirements and must be avoided in large volume manufacturing, even though certain biorefinery processes have been readily engineered on a sizeable scale, such as production of lactates from sugar‐derived lactic acid (LacA), alkyl glucoside surfactants from mono‐ and oligosaccharides, or biodiesel fuel from edible seed oils [ 1, 2]. In contrast, nonfood competitive materials, such as plant cell walls (lignocellulose), or oleaginous and keratinous waste are sustainable feedstocks for industrial chemical processing [ 4, 24, 25]. Examples of sustainable feedstocks include non‐food‐competitive waste products and side streams of forestry, horticulture and agriculture, vegetable oil refinery, animal husbandry, along with animal feather, wool, horns, and other indigestible fibrous proteins (keratinous feedstocks).
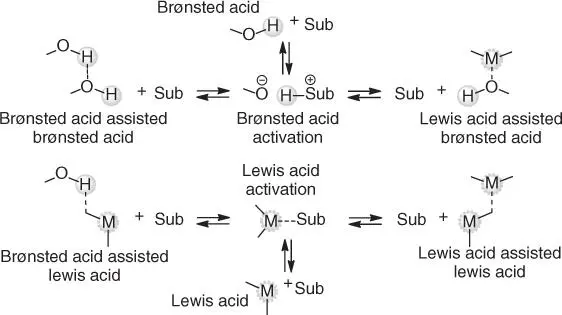
Scheme 2.1 Types of acid catalysts and acid‐catalyzed activations. Sub, substrate. H +, hydrogen ion; although Brønsted acids are conventionally denoted as H +, the hydrogen ion is known to form Lewis acid–Lewis base complexes, for instance, H 13O 6 +in aqueous solutions. M, electron‐deficient center of the Lewis acid catalyst. Source: Bodachivskyi et al. [ 4, 7].
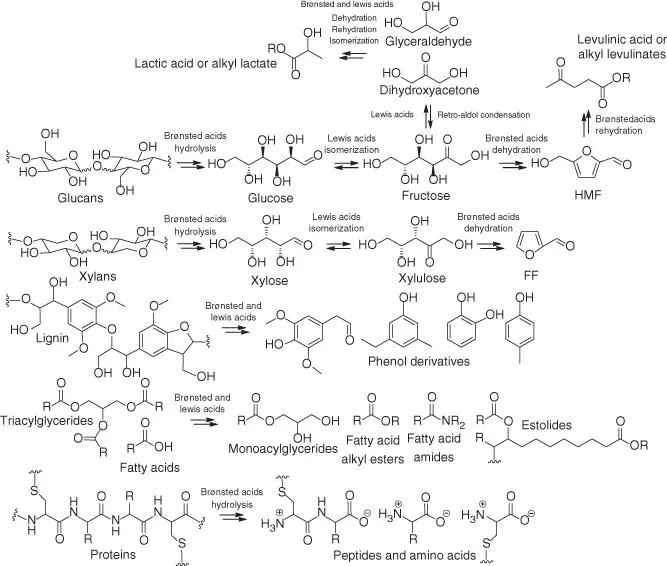
Scheme 2.2 Acid‐catalyzed valorization of biomacromolecules. R, H or organyl.
Although acid catalysis prevails in most processes with, for example, lignocellulose, it is not the only means that adds value to biomass, especially with noncellulosic substances. Sometimes, other strategies are more advantageous, brought about in large part because of the limitations of the chemistry under acid‐catalyzed conditions. For example, lipids, such as triacylglycerides and free fatty acids, can form manifold oleochemicals (functional products based on oleaginous materials) in the presence of an appropriate acid catalyst (Brønsted or Lewis) [ 1, 2, 10– 12]. In particular, acid‐catalyzed esterification of free fatty acids with low‐molecular‐weight alcohols is beneficial for the production of biodiesel fuel from waste cooking oils [ 26, 27]. However, base catalysts or transition metal catalysts are more efficient for the transformation of triacylglycerides into fuel products (fatty acid alkyl esters or varied hydrocarbons) and other functional oleochemicals [ 24, 26– 28]. Acid catalysts fare less well for such transformations [ 26, 27]. Additionally, the proteinaceous portion of biomass may be hydrolyzed into amino acids in solutions of concentrated Brønsted acids [13]. Nevertheless, the depolymerization of keratinous waste is often more efficient by other means than acid catalysis [25].
Polysaccharides, such as glucans and xylans, can be depolymerized into monomer sugars in the presence of Brønsted acid catalysts [ 4, 6]; such monosaccharides have many applications from organic synthesis [29]to energy sources for fermentation [30]. In the context of valorization chemistry toward platform molecules, for example, the depolymerization step is followed by the Lewis acid‐catalyzed aldose–ketose isomerization of monosaccharides, or their retro–aldol reactions, into other derivative carbohydrates [ 4, 7]. These intermediate products are typically convertible into furan‐type molecules or carboxylic acid derivatives under suitable processing parameters (catalyst, solvent, and reaction temperature). Indeed, the reaction conditions can be determinative of the selectivity toward specific types of products [ 4, 7].
Apart from carbohydrate polymers, lignin is known to transform into a range of useful phenol derivatives under acid‐catalyzed conditions, but the overall role of the catalysis is less well understood for these processes [ 5, 8, 9]. These bio‐based molecules may potentially generate a renewable platform that produces large volumes of green replacements to crude oil‐based products, such as fuels, monomers for plastics, detergents, and other commodity products [ 1– 13]. In the rest of this chapter, we will concentrate only on those transformations where acid catalysts display notable advantages over other types of catalysts. Effectively, this approach focuses on lignocellulosic materials.
2.2 Acid‐Catalyzed Processing of Cellulosic Polysaccharides
The valorization of lignocellulose is an intricate chemical problem. The challenge relates largely to the rigid supramolecular structure of native plant cell walls, formed by inter‐ and intramolecular bonding of polysaccharides, lignins, and sometimes other macromolecules [31]. A common strategy to improve reactivity is to separate carbohydrate polymers and lignin [32]. Fractionation of biomass is performed on large scale during commercialized pulping processes and may also be conducted by the selective catalytic transformation of one particular class of biomacromolecules [ 16, 32– 35]. For example, a carbohydrate fraction can be recovered by catalytic hydrogenolysis of lignin into low‐molecular‐weight phenolic substances [33]. It is also possible to retain the lignin fraction by the direct acid‐catalyzed processing of polysaccharides present in native biomass [ 34, 35]. To complicate matters, the acid‐catalyzed transformation of individual macromolecules is a complex cascade of reactions that require both Brønsted acid and Lewis acid catalysts ( Scheme 2.2) [ 4, 7]. In addition, acidic catalysts may simultaneously promote side reactions of the products into undesirable by‐products, compromising selectivity of the targeted processes [ 4, 7]. Consequently, judicious selection of the catalysts and processing conditions are essential to ensure the efficient processing of biomass into targeted value‐added products. This section will deal with major developments in the processing of cellulosic substances.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












