Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
bThe substrate was subjected to sulfuric acid‐assisted ball milling.
cILs were diluted with water during processing to a total water up to 10–43 wt% based on the reaction system.
d t does not include the time for the dissolution of the substrate.
eSubstrate was treated with aqueous sodium hydroxide.
The rates and (often) selectivities of the chemical process improve if the process can be performed under homogeneous conditions. As mentioned above, cellulosic materials are essentially insoluble in aqueous systems and most common organic solvents. However, ionic liquids (ILs) in their many manifestations are potentially key to improved chemical transformations of cellulosic materials. ILs are a class of green solvents that consist solely of ions and, under certain conditions, are able to fully dissolve cellulosic polysaccharides [ 48, 60, 61]. This ability enables significant progress toward milder reaction conditions and better yields of some targeted products, making ILs outstanding reaction systems for the acid‐catalyzed valorization of native carbohydrates [ 4, 61]. For example, quaternary ammonium salts, especially imidazolium derivatives such as 1‐alkyl‐3‐methylimidazolium chloride ([C nmim]Cl, n = integer), have been effectively employed in the catalytic hydrolysis of cellulose and cellulosic biomass of diverse origin [ 34, 49, 62, 63]. A seminal study [34]explored transformations of polysaccharides into monomer sugars (glucose and xylose) in a range of ILs in the presence of hydrochloric acid, a Brønsted acid catalyst. The study identifies that the molecular formula of ILs substantially influences the reaction outcomes, mostly related to the solubility of the substrate. There is an apparent need for the chloride anion to coordinate hydroxyl groups and to disrupt the extensive hydrogen bonding between polysaccharides, thus promoting their dissolution. In contrast, non‐coordinating, or weakly coordinating, anions, such as tetrafluoroborate (BF 4 −), nitrate (NO 3 −), bromide (Br −), and trifluoromethanesulfonate (triflate, OTf −), dissolve cellulose only poorly, slowing or preventing reactions from taking place [ 34, 64]. The cation component of the ILs also influences the chemical reactivity of the cellulose in ILs: imidazolium salts or alkylpyridinium‐based solvents with longer alkyl chains reduce the solubility of cellulose in such ILs, hampering the reactivity [ 58, 64]. Interestingly, imidazolium ILs with acetate (OAc −) and dimethylphosphate ((MeO) 2P(O)O −) counterions, which are excellent solvents for cellulose [64], provide zero yield of glucose after exposure to intended hydrochloric acid‐catalyzed processing of cellulose [34]. This is likely because of the reaction between the strong Brønsted acidic hydrochloric acid and the conjugate base of weaker acids, leading to formation of imidazolium chloride and a weaker Brønsted acid (acetic acid or dimethylphosphoric acid) that is unable to promote the hydrolysis under the selected processing conditions [62].
Another important finding is that acid‐catalyzed conversion of cellulosic biomass in IL media can be improved and effectively promoted by the gradual addition of water [34]. The optimized method provides impressive yields of glucose (up to 87 mol% based on the glucose content in the substrate) and xylose (up to 79 mol% based on the xylose component of the substrate) in [C 2mim]Cl solvent in the presence of hydrochloric acid catalyst at 105 °C, whose mixture was gradually diluted with 43 wt% of water ( Table 2.1). Previous methods, which were based on the processing of cellulose in [C 4mim]Cl without addition of water, provided significantly lower yields of monosaccharides [48]. Although glucose is the terminal product of hydrolysis chemistry of cellulose, the hydrolysis of cellulose proceeds predominantly into glucose oligomers (cellotetraose, cellotriose, and cellobiose) from which glucose emerges ( Scheme 2.4) [49]. There is also evidence that water suppresses the subsequent acid‐catalyzed conversion of saccharides into furanoids and by‐products in ionic solvents [ 34, 49, 62], thereby enhancing the yields of the desirable monosaccharides. The addition of water enhances the high yielding and selective transformation of polysaccharides into monomer sugars in ILs: it helps to promote the formation of monosaccharides and suppresses unwanted processes. However, care must be taken as to the timing of the addition of water. If this is done at the start of the process, then the substrate can remain undissolved because of the negative influence of the added water on the ability of ILs to dissolve biomass [ 34, 62]. Finally, the processing of lignocellulose in imidazolium ILs leaves a lignin‐rich residue as the unreacted portion that can be potentially employed for subsequent valorization [34]. Table 2.1provides processing conditions and outcomes of several instances, as described above.
ILs are highly tunable systems, and their physical and chemical characteristics can be modulated for specific tasks, providing high levels of flexibility for acid‐catalyzed processing [ 65– 67]. It is possible to design acidic ILs, which can simultaneously act as a solvent and catalyst, enabling the dissolution of substrates (solvent effect) and their subsequent conversions (catalyst effect) [ 49, 61, 63, 66, 67]. Such approaches potentially eliminate the need for highly corrosive mineral acid catalysts, such as hydrochloric or sulfuric acid, potentially avoiding the associated technological downsides accompanying their use. In an interesting study involving mixed solvent systems [63], the use of ammonium salts functionalized with Brønsted acidic sulfonic acid groups and hydrogen sulfate anion was investigated. N , N , N ‐Triethyl‐ N ‐(3‐sulfopropyl)ammonium hydrogen sulfate showed exceptional activity as a cosolvent in [C 4mim]Cl. The hydrogen sulfate system provided the Brønsted acid catalyst for the hydrolytic processing of MCC, affording very high yields of low‐molecular‐weight carbohydrates (yields of total reducing sugars 99%). The yields were determined using a colorimetric method based on the interaction of reducing carbohydrates with dinitrosalicylic acid [68], which may be subject to errors because of the reaction of dinitrosalicylic acid with other cellulose‐derived reducing substances. Nonetheless, the reported yields are impressive. More recently, our group has probed the use of a mixed ionic solvent system formed by [C 4mim]Cl and biorenewable acidic deep eutectic solvents (DESs; DESs are eutectic mixtures of Brønsted and Lewis acids and bases, often forming ILs) formed from choline chloride (ChCl) and oxalic acid dihydrate [49]. This mixed ionic system afforded high yields of low‐molecular‐weight saccharides (glucose yield up to 55 wt% and xylose yield up to 40 wt%, based on the substrate; yields were determined by liquid chromatography–mass spectrometry analysis, providing unambiguous detection of targeted products [49]) after processing of non‐pretreated cellulose (eucalyptus and Pinus ) and cellulosic materials of terrestrial (corncobs) and marine origin (micro‐ and macroalgae, Table 2.1), avoiding the use of corrosive acids. We proposed that the catalytic activity of the DES is generated by Lewis acid‐assisted Brønsted acidity, owing to the complexation between Lewis acidic ChCl and Brønsted acidic oxalic acid [49]. It is worth mentioning that reactions in the cosolvent system required the addition of water after dissolution of the substrate to promote the hydrolysis into monomer sugars, similar to the previous instances [34].
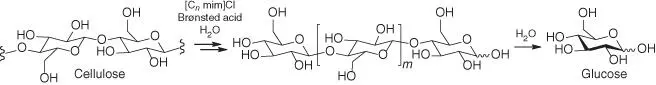
Scheme 2.4 Acid‐catalyzed hydrolysis of cellulose into low‐molecular‐weight carbohydrates in ILs via oligosaccharides. n, integer; m, 0, 1, and 2 for cellobiose, cellotriose, and cellotetraose, respectively.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












