Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
There is also a great deal of interest in zeolite‐catalyzed conversions of cellulosic saccharides into α‐hydroxy acids and esters [ 105– 108]. For example, Ga‐doped Zn/H‐nanozeolite Y catalysts are active in the transformation of MCC in supercritical methanol, yielding methyl lactate (MLac) as the major product (yield 58%, Table 2.2) and small amounts of methyl‐2‐methoxypropionate (MMP, yield 13%, Table 2.2) and MLev (5%, Table 2.2) [105]. Mesoporous Zr‐SBA‐15 silicate was reported to catalyze the transformation of cellulose in supercritical aqueous ethanol (95%), providing moderate yields of ethyl lactate (ELac, yield 30%), ethyl‐2‐hydroxybutanoate (EHB, yield 14%), and ELev (yield 2%, Table 2.2) [106]. A Sn‐beta zeolite has been employed in the valorization of the carbohydrate‐rich microalga Scenedesmus sp. into LacA in an aqueous ForA solution (in this instance, formic acid is a catalyst, not a product) [107]. The catalytic system enabled selective and high‐yielding conversion of algal sugars into LacA in 83% yield under optimal conditions (210 °C, two hours, Table 2.2). It is proposed that ForA helps to degrade the algal cell walls, whereas the Sn‐beta catalyst promotes the retro–aldol reaction of glucose and other reactions leading to LacA [107]. Most likely, the reported excellent yields relate to the ease of the depolymerization of structurally branched algal sugars under acid‐catalyzed conditions.
2.3 Acid‐Catalyzed Processing of Lignin
Lignin is a promising source of aromatic polymers for the commercial production of bulk chemicals [ 8, 9]. Until recently, developments in lignin refineries have somewhat lagged behind, in comparison to the processing of carbohydrates, and as any emerging field, it lacks deep fundamental understanding of the chemistry. It is considered that lignin is originated in plant cells by the polymerization of sinapyl alcohol, coniferyl alcohol, and sometimes p ‐coumaryl alcohol, which are addressed to the formation of S‐units, G‐units, and H‐units, respectively [ 109, 110]. These basic units are chemically bonded together by various types of C–O (β‐O‐4, α‐O‐4, and 4‐O‐5) and C–C (β‐5, 5‐5, β‐1, and β‐β) linkages, as portrayed in Scheme 2.7, and their ratio usually varies for different plants [ 109, 110]. Among them, the β‐O‐4 motif is the most common in nature (up to 70% of the total) [ 8, 32]. Various strategies have been devised for the valorization of commercially available lignin, but the most useful methods to date are based on the pyrolytic conversion of aromatic polymers into a range of phenol derivatives (e.g. phenol, catechol, and cresols), gaseous products (e.g. CO, H 2, and CH 4), and solid char [ 9, 111]. Pyrolysis is frequently promoted by acidic catalysts, such as metal chlorides and zeolites [9], and although some of these can be considered to be sustainable technologies, here, we omit these methods and discuss only acid‐catalyzed processing at moderate temperatures (pyrolysis of biomass is discussed in Chapters 6 and 7). Transition metal‐catalyzed reduction of lignin into low‐molecular‐weight derivatives is also of great industrial interest, especially in efforts to develop continuous processing of biomass [ 109, 112].
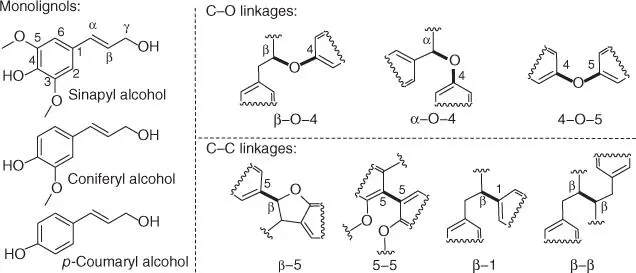
Scheme 2.7 Monolignols and possible linkages in lignin (specific bond types are highlighted in bold).
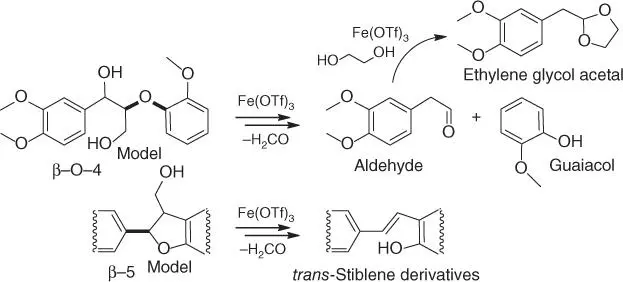
Scheme 2.8 Proposed acid‐catalyzed cleavage of lignin models (specific bond types are highlighted in bold). Reaction conditions: 1,4‐dioxane (solvent), ethylene glycol (4 eq., based on the substrate), Fe(OTf) 3(10 mol%, based on the substrate), 140 °C, 15 minutes. Source: Based on Deuss et al. [113].
In distinct contrast to cellulose, lignin is a structurally branched biopolymer that is soluble in many organic solvents and can be cleaved under relatively mild conditions. The principal problem in the processing of lignin is that depolymerization products tend to rapidly repolymerize in the process forming thermodynamically stable C–C linkages [ 8, 33, 109]. For example, reactions of various model substances in 1,4‐dioxane in the presence of metal triflates demonstrated that β‐O‐4 lignin models can be fully depolymerized into derivative aromatic aldehyde with a carbonyl group at the β‐position and guaiacol (140 °C, 15 minutes, Scheme 2.8) [113]. The reaction may also occur toward a derivative ketone, analogous to Hibbert ketones (products of the acidolysis of lignin) [114]; this pathway has not been confirmed ( Scheme 2.8) [113]. In contrast, a β‐5 model undergoes ring‐opening reactions into the corresponding trans ‐stilbene derivatives ( Scheme 2.8), while a β‐β model undergoes only epimerization in the acidic media ( Scheme 2.8does not show this transformation). Fe(OTf) 3was found to possesses optimal activity for the cleavage of β‐O‐4 linkages. It has been hypothesized that the activity of metal triflates relates to the generation of Brønsted acidic triflic acid in the reaction media [113]. This is nonetheless unlikely, as metal triflates are known to generate Brønsted acidity via an assisted acidity mechanism ( Scheme 2.1), not by the hydrolysis into triflic acid [ 7, 21, 103]. Additionally, targeted studies have demonstrated that triflic acid is not causative [ 115, 116]. Another important observation in the study [113]is that repolymerization can be effectively suppressed by the addition of ethylene glycol to form stable acetals from reactive products. The subsequent acid‐catalyzed depolymerization of varied substrates under defined optimal conditions (solvent dioxane, catalyst Fe(OTf) 3, 140 °C, 15 minutes [ 113, 117]) showed the relationship between the amount of produced monomers and the β‐aryl ether content of lignin. The conversion of lignins obtained by organosolv processes (extraction of the product with low molecular weight alcohols) using substrates with high β‐aryl ether content led to improved yields of phenolic acetals (up to 36 wt% of monomers), while the processing of industrial kraft lignins (lignins obtained by treatment of cellulose with aqueous Na 2S and NaOH) with their high content of C–C linkages provided lower yields of product (around 10 wt%) [117]. This apparently relates to the repolymerization of labile C—O bonds into stable C—C linkages during the kraft pulping process. Although there are isolated instances where the ratio between C–O and C–C linkages is not so clearly linked to reaction outcomes [109], it is clear that the composition of the substrate influences the overall progress of the reaction. The selection of lignin types is therefore essential for the development of specific catalytic methods.
There has been some effort to promote the valorization of lignin in ILs. The transformations of varied β‐O‐4 model compounds in common imidazolium salts in the presence of Brønsted or Lewis acidic catalysts demonstrate good activity of such systems for the hydrolytic processing of the substrates [ 118– 121]. The presence of some form of Brønsted acidity is requisite for the cleavage of β‐O‐4 bonds, whether this is achieved via the addition of protic acids, hydrolysis of metal salts, or by Lewis acid‐assisted Brønsted acidity. Hydrolysis and reduction of reactive intermediates into stable alcohol derivatives may be accomplished by the simultaneous hydrolysis/reduction of 2‐(2‐methoxyphenoxy)‐1‐phenylethanone into guaiacol and 2‐phenylethanol (yields nearly 60% each [122]) in the mixed ionic system comprising 1‐butyl‐2,3‐dimethylimidazolium bis(trifluoromethanesulfonyl)imide, the Brønsted acidic IL 1‐(4‐sulfobutyl)‐3‐methylimidazolium triflate, and IL‐stabilized ruthenium nanoparticles (a catalyst for the hydrogenation step) [122]. These studies have improved fundamental understanding of the processes involved [ 118– 122]; translation of such model reactions to the conversion of macromolecular lignin is yet to be done.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












