The acini are the primary secretory organs but the saliva is modified as it passes through the intercalated, striated, and excretory ducts before being discharged into the mouth and oropharynx ( Figure 1.14). The lobules also contain significant amounts of adipose tissue particularly in the parotid gland. The proportion of adipose tissue relative to excretory acinar cells increases with age.
In the human parotid, the excretory acini are almost entirely serous. In the submandibular gland, again, the secretory units are mostly serous but there are additional mucous tubules and acini. In some areas, the mucinous acini have crescentic “caps” of serous cells called serous demilunes. In the sublingual gland, the acini are almost entirely mucinous although there are occasional serous acini or demilunes.
The serous cells contain numerous proteinaceous secretory (zymogen) granules. These granules contain high levels of amylase. In addition, the secretory cells produce kallikrein, lactoferrin, and lysozyme. In mucous cells, the cytoplasm is packed with large pale secretory droplets.
Initially the secretory acini drain into intercalated ducts. These ducts function mainly to conduct the saliva but they may also modify the electrolyte content and secrete immunoglobulin A. The intercalated ducts drain into striated ducts that coalesce into intralobular and extralobular collecting ducts. The intercalated duct cells are very active metabolically, and they transport potassium and bicarbonate into saliva. They reabsorb sodium and chloride ions so that the resulting saliva is hypotonic. They also secrete immunoglobulin A, lysozyme, and kallikrein. The immunoglobulin is produced by plasma cells adjacent to the striated duct cells, and it is then transported through the epithelial lining into the saliva. The main collecting ducts are simple conduits for saliva and do not modify the composition of the saliva.
Myoepithelial cells are contractile cells closely related to the secretory acini and much of the duct system. The myoepithelial cells lie between the basal lamina and the epithelial cells. Numerous cytoplasmic processes arise from them and surround the serous acini as basket cells. Those associated with the duct cells are more fusiform and are aligned along the length of the ducts. The cytoplasm of the myoepithelial cells contains actin myofilaments that contract as a result of both parasympathetic and sympathetic activity. Thus, the myoepithelial cells “squeeze” the saliva out of the secretory acini and ducts and add to the salivary secretory pressure.
Although the parotid capsule is a continuous structure covering the superficial and deep aspects of the gland, it becomes very attenuated where the gland envelopes the branches of the facial nerve. This is of some significance in parotid surgery as when peeling the gland off the branches of the nerve there may be none or a very thin capsule separating the gland from the nerve.
There is a continuous low background saliva production that is stimulated by drying of the oral and pharyngeal mucosa. A rapid increase in the resting levels occurs as a reflex in response to masticatory stimuli including the mechanoreceptors and taste fibers. Other sensory modalities such as smell are also involved. The afferent input is via the salivatory centers that are themselves influenced by the higher centers. The higher centers may be facilitory or inhibitory depending on the circumstances. The efferent secretory drive to the salivary glands passes via the parasympathetic and sympathetic pathways. There are no peripheral inhibitory mechanisms.
Cholinergic nerves (parasympathetic) often accompany ducts and branch freely around the secretory endpieces (acini). Adrenergic nerves (sympathetic) usually enter the glands along the arteries and arterioles and ramify with them. Within the glands, the nerve fibers intermingle such that cholinergic and adrenergic axons frequently lie in adjacent invaginations of a single Schwann cell (Garrett and Kidd 1993). Secretion and vasoconstriction are mediated by separate sympathetic axons whereas a single parasympathetic axon may, through serial terminals, result in vasodilatation, secretion, and constriction of myoepithelial cells.
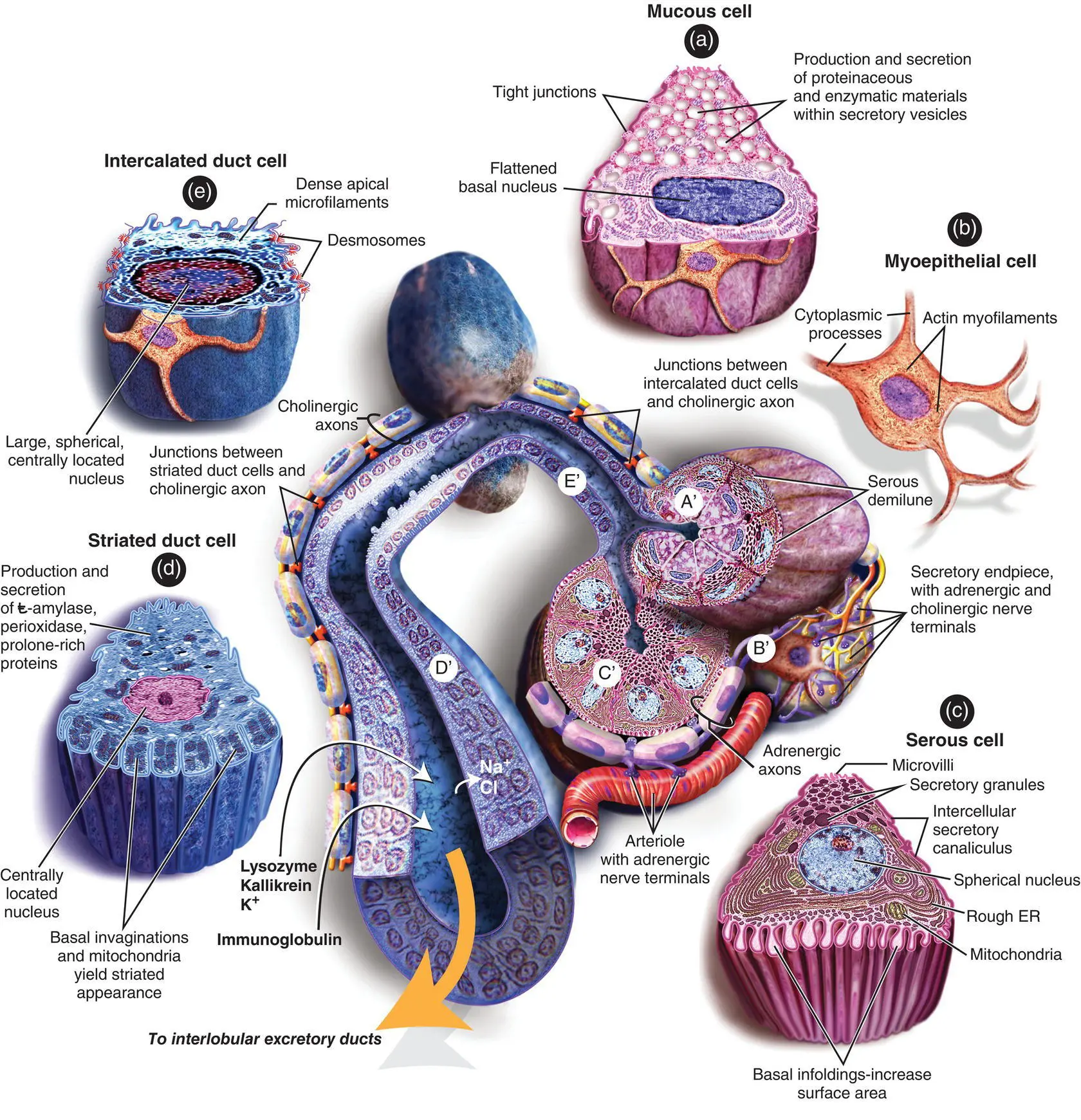
Figure 1.14. Diagram showing the histology of the major components of the salivary glands.
Secretory endpieces are the most densely innervated structures in the salivary glands. Individual acinar cells may have both cholinergic and adrenergic nerve endings. The secretion of water and electrolytes, which accounts for the volume of saliva produced, results from a complex set of stimuli which are largely parasympathetic. The active secretion of proteins into the saliva depends upon the relative levels of both sympathetic and parasympathetic stimulation.
Although the ducts are less densely innervated than secretory acini, they do influence the composition of the saliva. Adrenal aldosterone promotes resorption of sodium and secretion of potassium into the saliva by striated ductal cells. Myoepithelial cell contraction is stimulated predominantly by adrenergic fibers although there may be an additional role for cholinergic axons.
The cholinergic parasympathetic nerves release acetylcholine that binds to M3 and to a lesser extent M1 muscarinic receptors which result in the secretion of saliva by the acinar cells in the endpieces of the duct trees. The sympathetic nerves release noradrenaline that results in the release of stored protein from both the acinar cells and the ductal cells. There is also cross talk between the calcium and cyclic AMP intracellular pathways. Additionally, other non‐adrenergic and non‐cholinergic neuropeptides released from the autonomic nerves evoke saliva secretion and parasympathetically derived vasointestinal peptide acting through endothelial cell‐derived nitric oxide. These neuropeptides play a role in the reflex vasodilatation that accompanies salivary secretion (as seen dramatically in Frey syndrome). Neuronal type, calcium activated, soluble nitric oxide within salivary cells seems to play a role in mediating salivary protein secretion in response to autonomimetics. The fluid secretion involves aquaporin 5 and the extent to which its expression on apical acinar cell membranes is upregulated by cholimimetics remains obscure (Proctor and Carpenter 2007).
Although embryologically the parotid consists of a single lobe, anatomically the facial nerve lies in a distinct plane between the anatomical superficial and deep lobes.
The parotid capsule is attenuated and incomplete where the gland lies in intimate contact with the branches of the facial nerve.
There are fixed anatomical landmarks indicating the origin of the extracranial facial nerve as it leaves the stylomastoid foramen.
The lower pole of the parotid gland is separated from the posterior pole of the submandibular gland by only thin fascia. This can lead to diagnostic confusion in determining the origin of a swelling in this area.
The relationship of the submandibular salivary duct to the lingual nerve is critical to the safe removal of stones within the duct.
Great care must be taken to identify the lingual nerve when excising the submandibular gland. The lingual nerve is attached to the gland by the parasympathetic fibers synapsing in the submandibular (sublingual) ganglion.
The sublingual gland may drain into the submandibular duct or it may drain directly into the floor of the mouth via multiple secretory ducts.
Case Presentation – Wait, What?
Читать дальше













