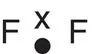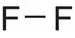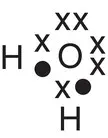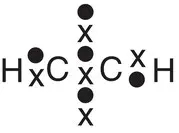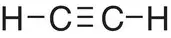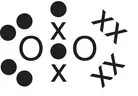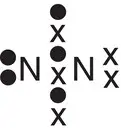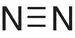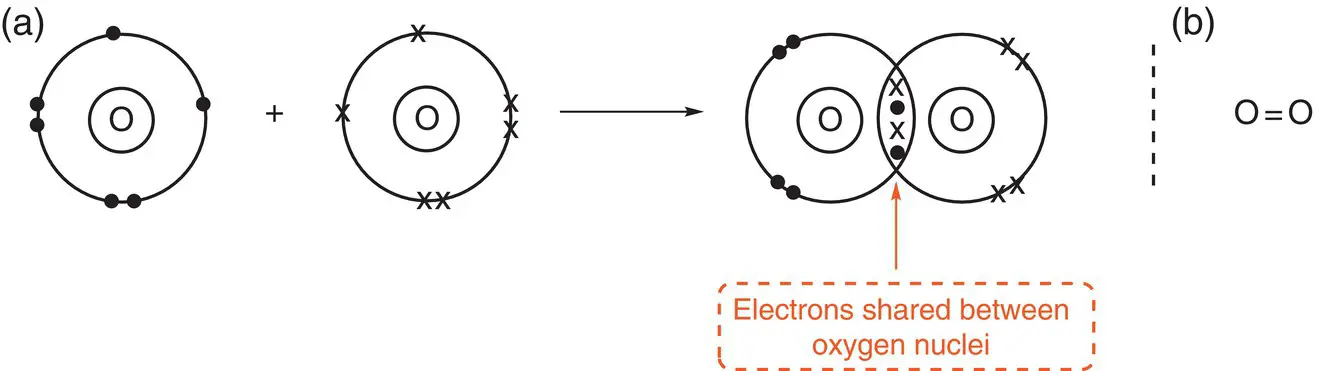
Figure 2.9(a) Formation of a double bond in oxygen, O 2; (b) an alternative representation of an oxygen molecule with a double bond.
The symbol π is the Greek letter pi. A π bond consists of a pair of electrons located between two covalently bonded atoms in a plane at 90° to the bond axis.
The oxygen atom has six outer electrons. The electron configuration is 1s 22s 22p 4. An oxygen atom requires two additional electrons to fill its outer shell and attain a complete octet of electrons as in the atom neon, the closest noble gas. Thus, in the formation of an oxygen molecule, each oxygen atom shares two of its outer electrons with another oxygen atom. There is therefore a total of four electrons bonding the oxygen atoms together. When four electrons are shared between two atoms, a double bond is formed, which is represented by a double horizontal line between the atoms in the bond.
In covalent bonding, the first two electrons to be shared form a sigma bond. If more than two electrons are shared, the electrons occupy pi bonds.
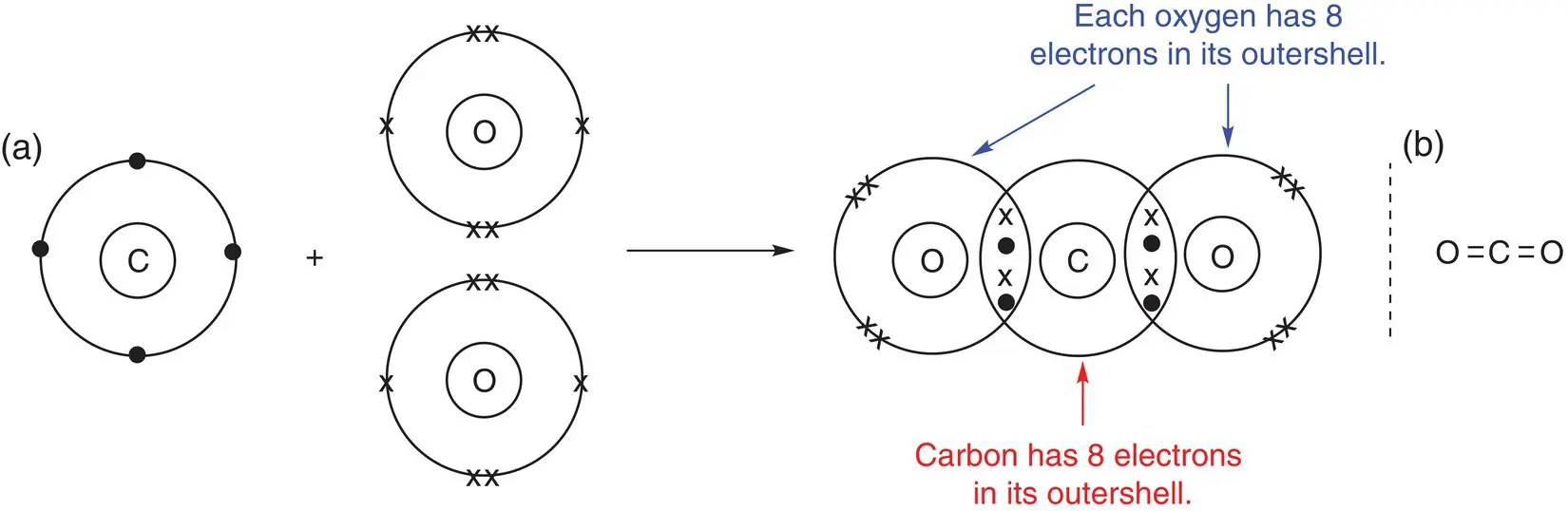
Draw a dot‐and‐cross diagram to show the bonding present in carbon dioxide, CO 2.
Carbon dioxide contains two elements: carbon and oxygen. Both are non‐metals; therefore, the type of bonding between them will be covalent, i.e. shared pairs of electrons. Carbon has four electrons in its outer shell and therefore needs to gain another four electrons to fill its outer shell. Oxygen has six electrons, so it needs to gain two more electrons. In addition, the name carbon dioxide gives a hint about the structure, with the ‘di’ showing that there are two oxygen atoms.
This information allows us to deduce that if carbon shares two electrons with each oxygen, each oxygen will have eight electrons in its outer shell and so will have a complete octet. Conversely, if oxygen shares two electrons each with carbon, carbon will have gained a share of four electrons in total, so it will also have a full outer shell. In the case of carbon dioxide, there are two pairs of electrons between each nucleus (a). Thus, there is a double bond between each carbon and oxygen atom. This is represented by a double line between the atoms (b).
For all structures containing covalent bonds, it is possible to work out the bonding and how the atoms are arranged. Some common molecules are shown in Table 2.1.
Table 2.1The names, molecular formulae, dot‐and‐cross diagrams, and display formulae of some commonly encountered covalently bonded molecules.
| Name and molecular formula |
Dot‐and‐cross diagram |
Display formula |
| Fluorine, F 2 |
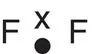 |
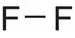 |
| Water, H 2O |
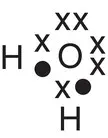 |
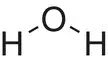 |
| Ammonia, NH 3 |
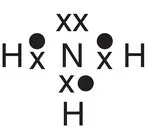 |
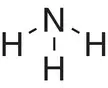 |
| Methane, CH 4 |
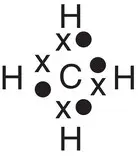 |
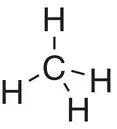 |
| Ethene, C 2H 4 |
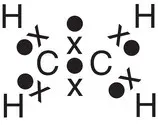 |
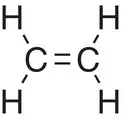 |
| Ethyne, C 2H 2 |
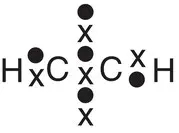 |
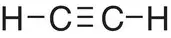 |
| Oxygen, O 2 |
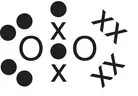 |
 |
| Nitrogen, N 2 |
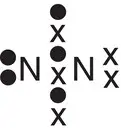 |
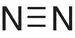 |
To ensure that you understand this topic, try to draw each of the molecules in Table 2.1using dot‐and‐cross diagrams and starting from the component atoms, accounting for all of the electrons shown.
In some of the molecules shown in Table 2.1, a pair of electrons is ‘left over’ or not involved in covalent bond formation. Examples of molecules where this occurs include ammonia (one pair of electrons on the nitrogen), water (two pairs of electrons on the oxygen), and nitrogen (one pair of electrons on each nitrogen). This pair of electrons is called a lone pair of electrons or, more colloquially, a lone pair . Such pairs of electrons can also undergo bonding to other species that can accept a pair of electrons, such as a positive hydrogen ion, H +. When both electrons in a covalent bond originate from the same atom, a dative covalent bond is formed. An example of this is ammonia (NH 3), which can form a dative covalent bond to a proton (H +) to form an ammonium ion (NH 4 +). The dot‐and‐cross diagrams showing the formation of a dative covalent bond in the ammonium ion are given in Figure a2.10. A dative covalent bond can be shown either as a line or as an arrow. The arrow is drawn such that it starts on the atom that has donated the electrons and finishes on the atom that has gained the pair of electrons, as shown in Figure 2.10b. Once formed, the dative covalent bond between the nitrogen and hydrogen atoms is chemically no different to the other nitrogen—hydrogen bonds in the ammonium ion.
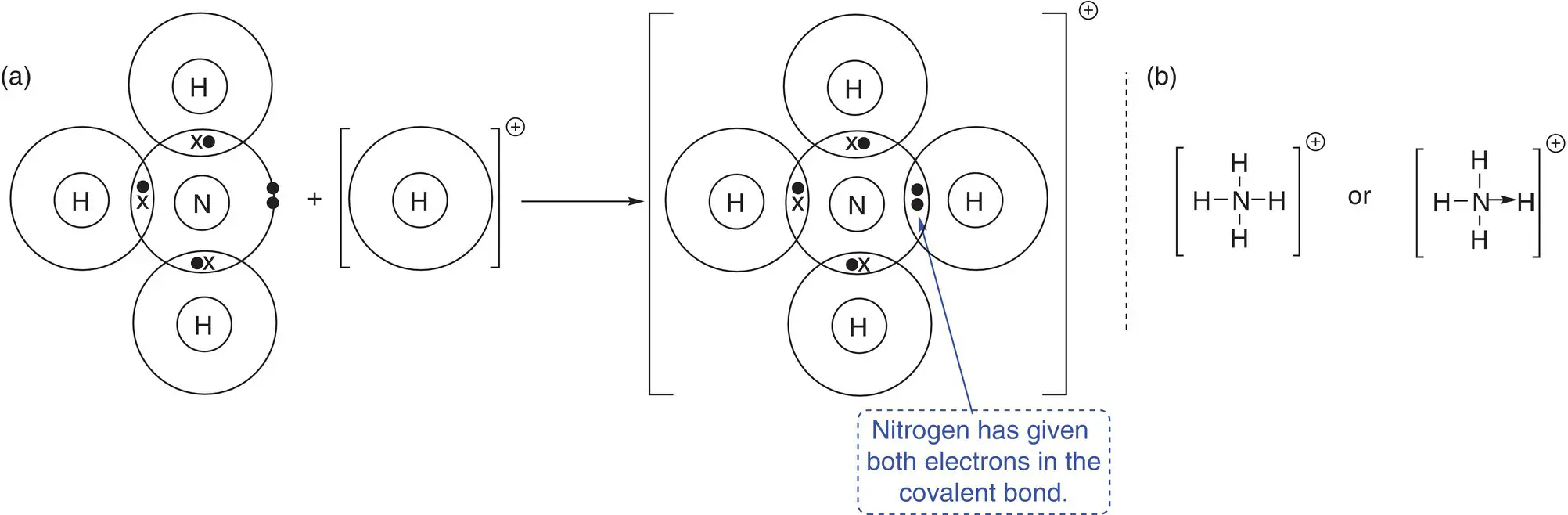
Figure 2.10(a) Bonding in the ammonium ion, NH 4 +. Note: only outer‐shell electrons are shown for clarity. (b) An alternative representation of bonding in the ammonium ion.
Читать дальше