Sodium chloride is an ionic compound that contains sodium ions (Na +) and chloride ions (Cl −). Sodium is in Group 1 and therefore has one electron in its outer shell. Chlorine is in Group 7 (Group 17) and has seven outer electrons. To have a full outer shell, sodium must lose one electron and chlorine must gain one more electron, as shown in Figure 2.5.
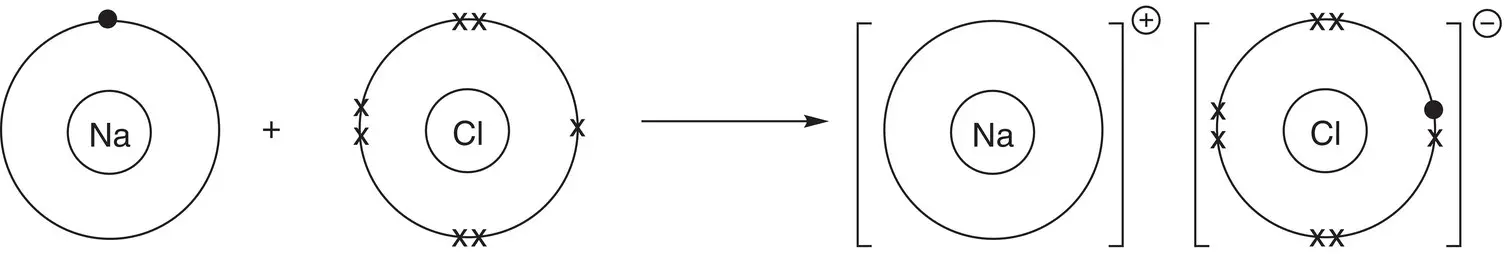
Figure 2.5Bonding in NaCl. Note: only outer‐shell electrons are shown for clarity.
When chlorine has a negative charge, the name changes to chloride . The same is true for fluorine (fluoride), bromine (bromide), and iodine (iodide).
In Figure 2.5, sodium's outer electrons are represented by dots and chlorine's outer electrons by crosses. By losing one electron to chlorine, sodium now has one fewer electron than protons and so has an overall positive charge. In contrast, chlorine has one more electron than protons, so it has an overall negative charge. The resulting ions are shown in square brackets, where the charge of each ion is at the top right, on the outside of the bracket.
This type of diagram, where electrons are represented by dots and crosses, is called a dot‐and‐cross diagram and sometimes a Lewis structure . Note that all electrons are identical to each other, regardless of whether they are drawn as dots or crosses.
The positive sodium ions and negative chloride ions are attracted to each other by electrostatic forces and are arranged in a regular lattice array. Figure 2.6shows the arrangement of ions in the sodium chloride lattice. Many millions of these units are linked together in a grain of salt ( Figure 2.7).
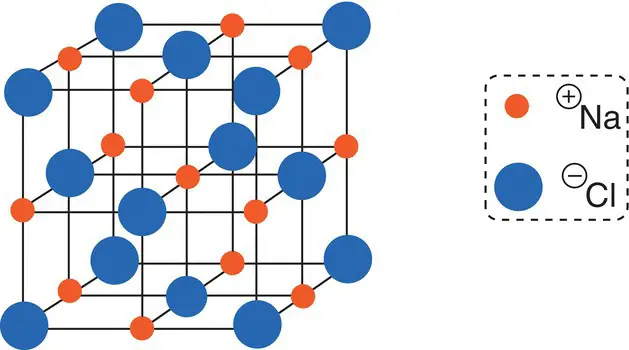
Figure 2.6The sodium chloride lattice. Source: Based on https://www.chemguide.co.uk/atoms/structures/ionicstruct.html.

Figure 2.7Salt, sodium chloride.
Source: Dr Philippa Cranwell.
Ionic bonding is very strong, and, as a result, ionic compounds or salts have similar physical properties. Ionic compounds usually have high melting and boiling points and are solids at room temperature. They form hard crystalline structures. When solid, they do not conduct heat or electricity; but when molten, they do, as the ions can move and carry a charge or transfer heat energy.
Ionic bonding occurs between metals and non‐metals, and there is a strong electrostatic interaction between the bonding partners.
Give the electronic configuration of the following atoms and the likely electron configuration and charge of the species formed by each atom upon ionisation. State the noble gas atom that has the same electron configuration as the ion formed.
1 potassium
2 chlorine
1 Potassium is an s block element in Group 1 of the periodic table. It is a metal with one outer electron. The electron configuration of potassium is 1s22s22p63s23p64s1. To gain a full outer shell, potassium tends to lose its outer electron and form a K+ ion with electron configuration 1s22s22p63s23p6. This electron configuration is the same as argon, Ar.
2 Chlorine is in Group 7 (Group 17) of the periodic table and has seven outer electrons with electron configuration 1s22s22p63s23p5. It is a non‐metal and tends to gain an electron to form the chloride, Cl−, ion with electron configuration 1s22s22p63s23p6. This electron configuration is the same as argon, Ar.
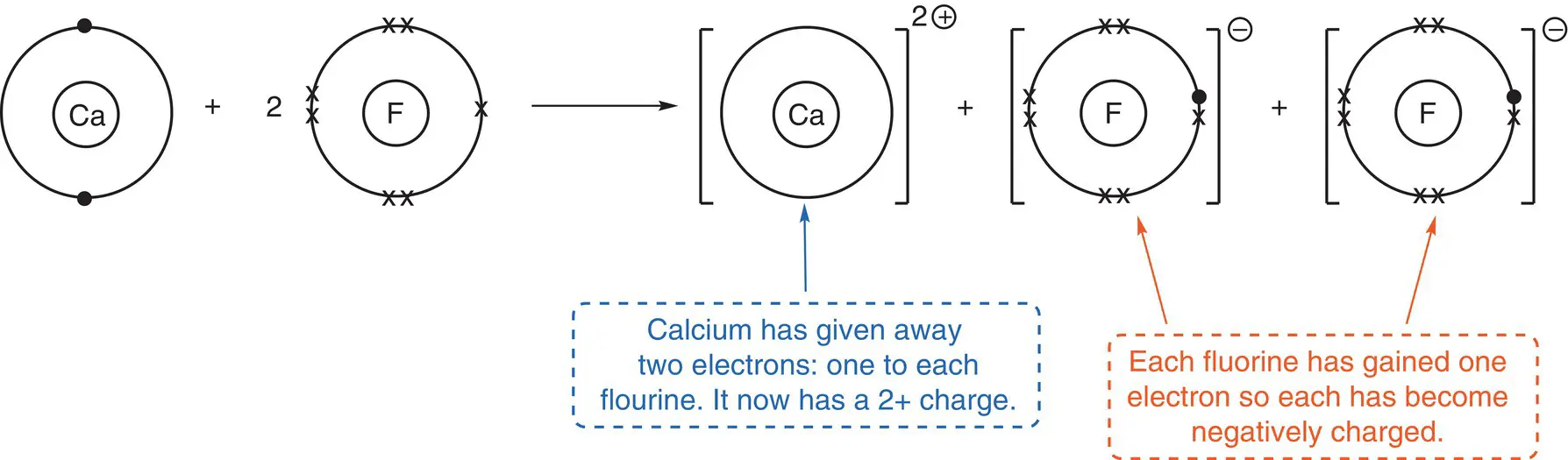
Draw the Lewis structure (dot‐and‐cross diagram) for calcium fluoride, CaF 2.
Calcium fluoride contains two elements: calcium and fluorine. Calcium is a metal on the left of the periodic table, in Group 2. Fluorine is a non‐metal and resides in Group 7 (Group 17). Because we have a metal and a non‐metal in the compound, the type of bonding present is ionic. Therefore, the main interaction between atoms will be electrostatic: i.e. a positive ion with a negative ion.
When asked a question about the type of bonding in a compound, the first thing to check is the types of elements that are bonded and whether they are metals or non‐metals.
As stated earlier, calcium is in Group 2. Therefore, to gain a full outer shell, it needs to lose two electrons. In doing this, it will have a 2+ charge.
Fluorine is in Group 7. Therefore, to gain a full outer shell, it needs to gain one electron. By doing this, it has a negative charge. To balance the two electrons given up by calcium, there need to be two fluorine atoms present that can each take one electron.
Covalent bonding occurs between two non‐metal atoms. In a covalent bond, each atom contributes one or more electrons to share with the other atom. The driving force behind covalent bonding is similar to that in ionic bonding: each element wants to achieve a full outer shell of electrons. In the case of covalent bonding, electrons are shared rather than lost or gained. The covalent bond itself is very strong; a large amount of energy is required to break a covalent bond.
Covalent bonding occurs between non‐metals. A covalent bond is a shared pair of electrons.
We will use the formation of the H—H bond in molecular hydrogen to explain covalent bonding. Hydrogen is an element that can undergo covalent bonding. In an atom of hydrogen, there is only one electron in a 1s orbital (the outermost shell). To achieve a full outer‐shell, hydrogen needs to have two electrons; therefore, one hydrogen atom shares its electron with that of another atom of hydrogen. The 1s orbitals containing the electrons overlap. This is shown in Figure 2.8a. In this figure, the electrons on each hydrogen are shown as a dot or a cross. When the electrons are shared, they are shown within the overlapping rings of the outermost shell. When two electrons are shared, they are said to form a bonding pair between the two atoms. One pair of electrons is one bond and can be represented by a single line, as in Figure 2.8b. In the case of hydrogen, H 2, there is one pair of electrons that is located between the two nuclei. This is called a (sigma) σ bond. Figure 2.8c is a representation of the electron cloud showing where the two electrons in a σ bond are located. This will be discussed further in Chapter 12.
The symbol σ is the Greek letter sigma. A σ bond consists of a pair of electrons shared between two covalently bonded atoms.
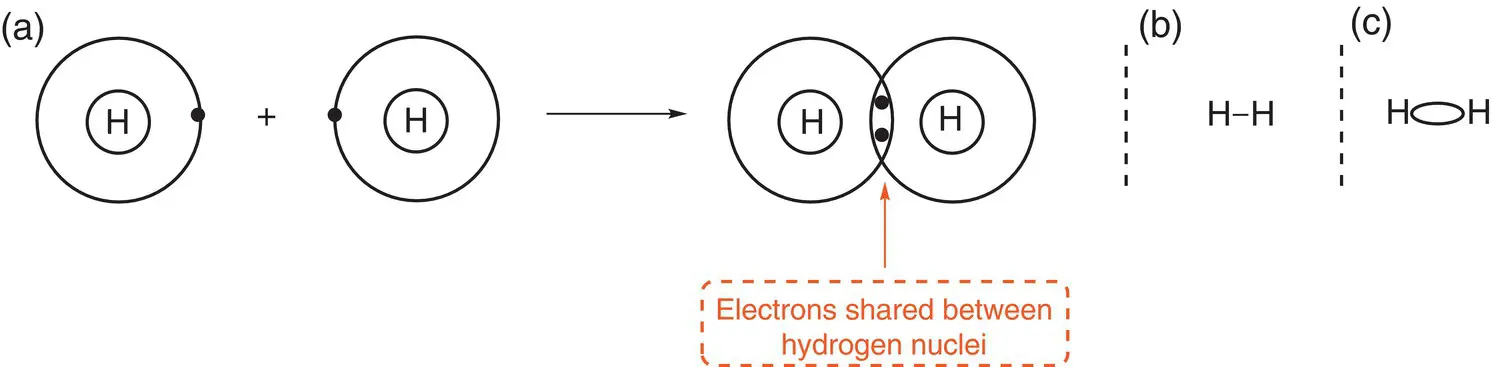
Figure 2.8(a) Formation of a single bond in hydrogen, H 2; (b) an alternative representation of a hydrogen molecule with a single bond; (c) a representation of the location of the electrons in a σ bond.
Although the electrons in the hydrogen atoms are shown as dots and crosses, all the electrons are identical.
The previous example for hydrogen, H 2shows the formation of a single covalent bond where two electrons are shared between the two atoms. In many cases, atoms must share more than two electrons. In such cases, a double or triple bond is formed. Double and triple bonds are made up of sigma (σ) and pi (π) bonds, which will be discussed further in Chapter 12. An example of a molecule where a double bond is present is oxygen, O 2. This is shown in Figure 2.9.
Читать дальше

















