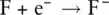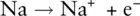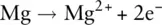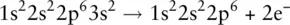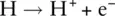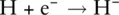Figure 2.1(a) Eight hydrogen atoms; (b) four hydrogen molecules.
Atoms of different elements can also bond together to form molecules. When two or more elements are chemically joined together a compoundis formed. Water, H 2O, carbon dioxide, CO 2, and ethanol, C 2H 5OH, are all examples of compounds.
An atom contains electrons, protons, and neutrons. A moleculeis formed when two (or more) atoms bond together.
Within chemistry, there are three main ways in which atoms can bond together to make larger units. These bonding types are called metallic , ionic , and covalent . The first part of this chapter explains the different types of bonding that can occur and the types of compounds formed.
When considering bonding, the driving factor that causes an atom to lose or gain electrons is the extra stability the atom gains when it has a full outer shell of electrons. For example, the noble gases in Group 8 (Group 18) are especially stable because all of their orbitals are filled with electrons. Other atoms in the s and p blocks of the periodic table react by losing, gaining or sharing electrons in order to have the same electron configuration as the nearest noble gas element. This tendency of elements to attain a full outer shell of electrons is called the octet rule because the noble gases Ne and Ar have eight outer electrons.
The word octet is derived from the Latin for the number eight, which is octo . Hence the octet rule refers to eight electrons.
Metallic bonding occurs in metals and alloys when metal atoms are bonded to other metal atoms. When metal atoms join together, their outer (valence shell) electrons are lost, leaving positively charged metal ions. The electrons form a ‘sea’ of negative charge surrounding the positive metal ions. The interaction between the sea of electrons and the positive metal centres holds the atoms together in the bulk metal. A representation of the bonding in sodium is shown in Figure 2.2. Sodium is in Group 1 and has only one outer electron that is easily lost. The positive sodium ions form a regular ordered array surrounded by the sea of negative electrons.
The valence shell is the outermost shell in an atom that contains electrons.
An ion is an atom that has lost or gained one or more electrons to form a charged species. A positive ion is called a cation, and a negative ion is called an anion.
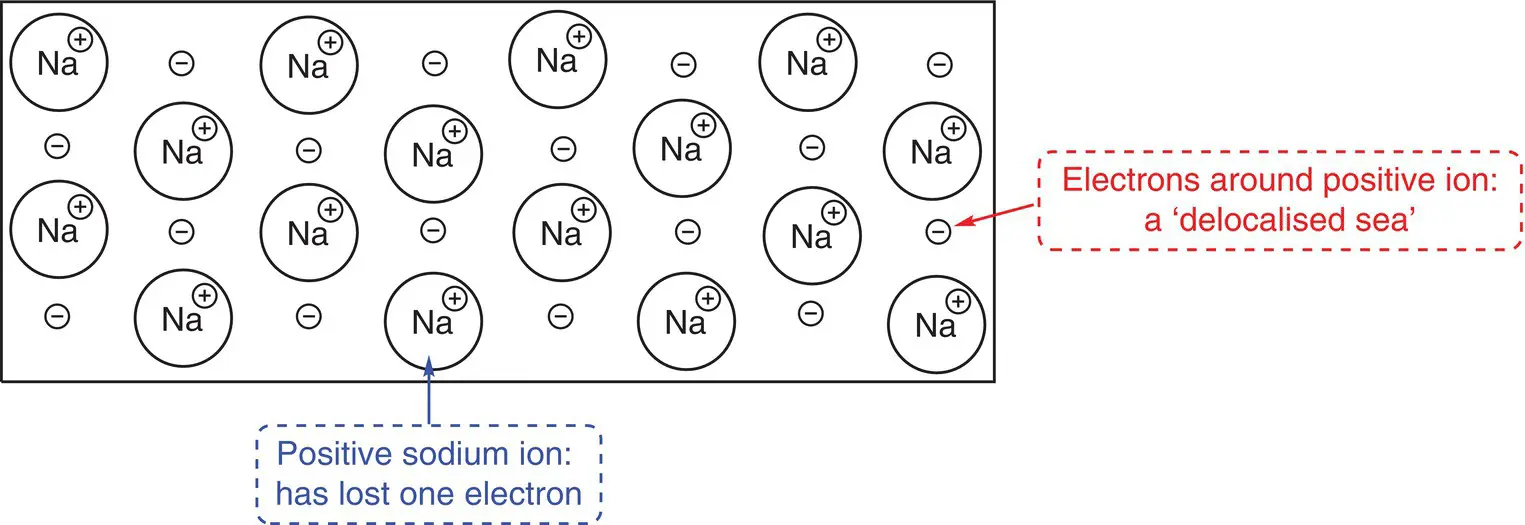
Figure 2.2Bonding in sodium metal.
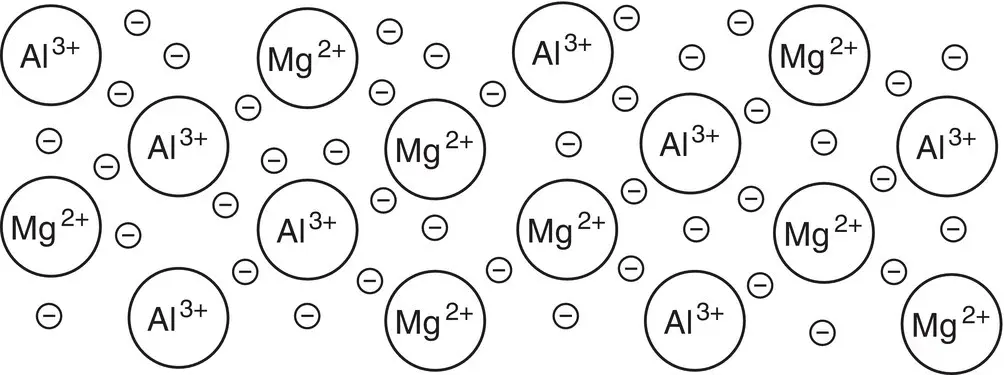
Explain and draw the bonding present in Magnox, an alloy of magnesium and aluminium that is used in the cladding of nuclear power reactors.
Magnesium is in Group 2, so it can lose two electrons. Therefore, each magnesium atom contributes two electrons to the sea of charge. Aluminium is in Group 3 and has three valence electrons that contribute to the sea of electrons. If we use this information, we can suggest what the bonding in the alloy may look like.
Ionic bonding occurs between a metal and a non‐metal. An ionic bond is an electrostatic interaction between two oppositely charged ions that are attracted to each other. Ions are formed when atoms lose or gain electrons, so they have either a positive or a negative charge. The metal ion always has a positive charge and the non‐metal ion a negative charge.
An example of this behaviour is shown by fluorine, which has the electron configuration 1s 22s 22p 5. When a fluorine atom gains one more electron, it has the electron configuration of neon, i.e. 1s 22s 22p 6 .It now has a charge of −1 because it has gained one negatively charged electron and becomes a fluoride ion, F −.
The process can be represented by the equation:
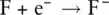
The electron arrangements in the fluorine atom and fluoride ion are shown in Figure 2.3.
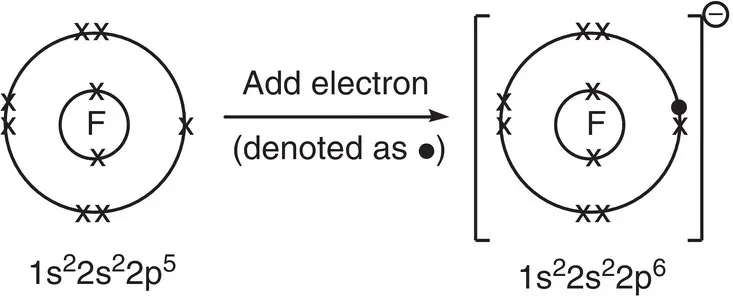
Figure 2.3Addition of an electron to a fluorine atom to generate a fluoride ion. Electrons in the fluorine atom are represented by x.
Note that the neutral F atom is called fluorine , as in the element, and the negatively charged F −ion is called fluoride . This is the form of the element found in toothpaste, for example.
To form a positive ion, the atom must lose an electron. Sodium in Group 1 has just one outer electron. Sodium attains an octet of eight outer electrons by losing this single electron. It is more favourable for sodium to lose this single electron and become a Na +ion than to gain an extra seven electrons. Sodium therefore loses an electron to attain the electron configuration of neon, as shown in Figure 2.4.
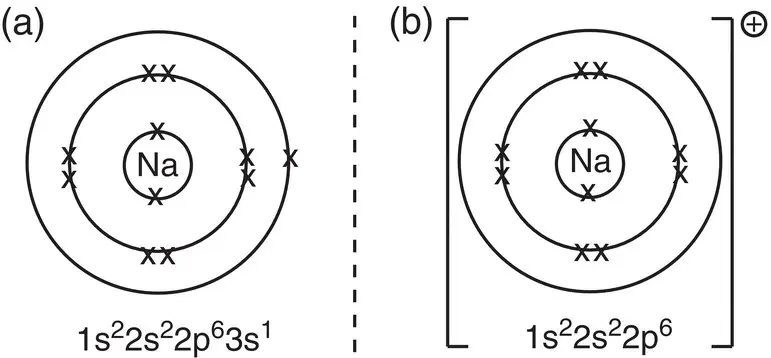
Figure 2.4Arrangement of electrons in (a) a sodium atom; (b) a sodium ion.
The process can be represented by the equation:
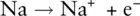
The element to the right of sodium in the periodic table is magnesium, Mg, and an atom of magnesium has two outer electrons (1s 22s 22p 63s 2). To attain a full outer shell, magnesium must lose both its 3s electrons. When this occurs, a Mg 2+ion is formed:
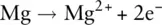
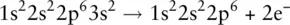
The process of losing or gaining an electron to form an ion is called ionisation .
Metal atoms always lose electrons on ionisation to form positive ions, whereas non‐metals gain electrons to form negative ions.
Elements in the first row of the periodic table (i.e. hydrogen and helium) cannot obtain an octet of electrons as they have a single s orbital that can only hold a maximum of two electrons. They therefore have a full outer shell when they have just two electrons in the 1s orbital. A helium atom has a full outer shell and so has no tendency to lose or gain electrons and therefore does not readily form ions. However, hydrogen has just one outer electron. It can either lose this electron and form a H +ion or gain an electron to form a H −ion. The H −ion is called a hydride ion.
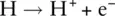
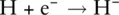
When a positively charged cation and a negatively charged anion are formed, an electrostatic interaction exists between the oppositely charged ions. This interaction is the basis of ionic bonding, and the attractive force between the ions is similar to that experienced between the north and south poles of a magnet. These attractive forces extend among all the cations and anions in a structure to give an ionic lattice . An ionic lattice is a regular three‐dimensional array of positive and negative ions extending throughout the structure. The attractive forces between the oppositely charged ions are very large; therefore, ionic bonds are very strong, and ionic lattices are difficult to break down. The bonding in sodium chloride can be used as an example of ionic bonding.
Читать дальше