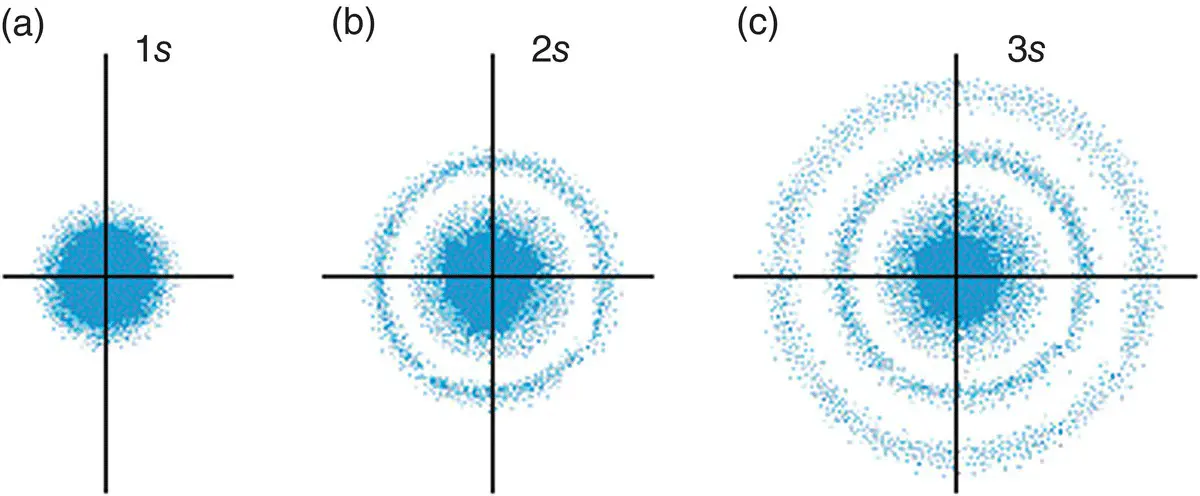
Figure 1.6(a) The 1s orbital; (b) the 2s orbital; (c) the 3s orbital.
Source: Redrawn from https://www.quora.com/What‐is‐a‐simple‐explanation‐of‐the‐Quantum‐mechanical‐model‐of‐the‐atom.
Elements that either have fully or partially filled s orbitals in their outer shells are called s‐block elements. These elements occupy Groups 1 and 2 of the periodic table.
Each s orbital can contain a maximum of two electrons. The second element in the periodic table, helium, has a total of two electrons. Both of the electrons in helium are therefore in the 1s orbital because the s orbital can hold up to two electrons and this is the lowest‐energy orbital. To allow both negatively charged electrons to occupy the same space and not fly apart, the electrons have opposite spins. This creates a very tiny magnetic field about each electron, causing them to behave as magnets with opposite polarity, and so holds them together in the orbital.
The concept of spin is beyond the scope of this textbook. But when representing electrons in orbitals, if there are two electrons in one orbital, make sure they are represented by arrows (or half arrows) pointing in opposite directions: i.e. one pointing up and one pointing down, as in Fig. 1.10.
The second principal quantum shell has one s orbital and three p orbitals. p orbitals are shaped like a dumb‐bell or figure of eight (see Figure 1.7). There are three p orbitals in each quantum shell, where n is greater than 1, and each p orbital points in a different direction and is at 90° to the other two. For simplicity, they are generally drawn aligned along the x, y, and z axes. The p orbitals in the second shell are named 2p x, 2p y,and 2p z.
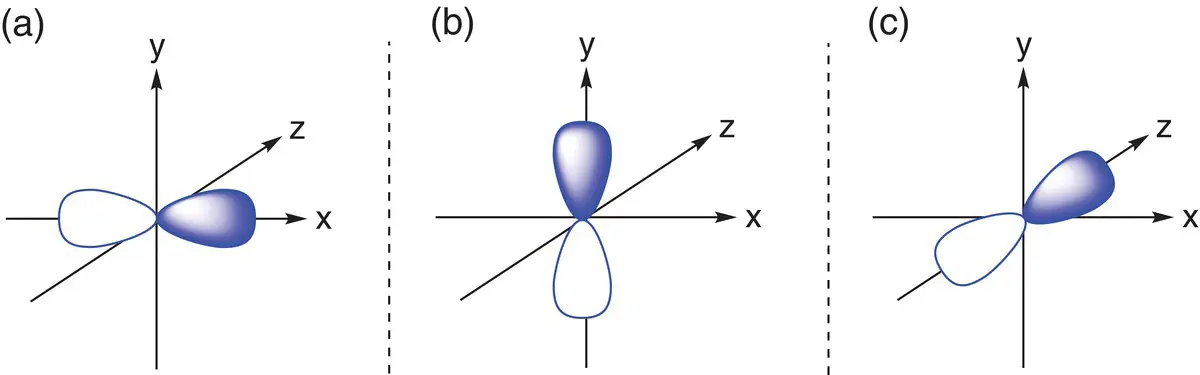
Figure 1.7(a) The 2p xorbital; (b) the 2p yorbital; (c) the 2p zorbital.
Just like s orbitals, p orbitals can only hold a maximum of two electrons. However as there are three p orbitals per energy level, there can be a maximum of six p electrons in total in a p sub‐shell. Elements that have filled or partially filled p orbitals in their outer shells are found in the p block of the periodic table, which consists of Groups 3–8 (also known as Groups 13–18).
The third principal quantum shell in an atom contains an s orbital (3s), three p orbitals (3p), and five d orbitals (3d). The shapes of the d orbitals are shown in Figure 1.8. These are slightly more complex than p orbitals and again point in different directions along the x, y, and z axes. Four of the d orbitals have a similar shape (although three have lobes that point between the axes and the fourth has lobes that point along the axes). The fifth d orbital (d z2) has a dumb‐bell shape with a donut ring around the middle. Again, each d orbital can hold a maximum of 2 electrons, so the maximum number of d electrons in any one energy level is 10. Elements that have outer electrons in d orbitals are found in the d block of the periodic table.
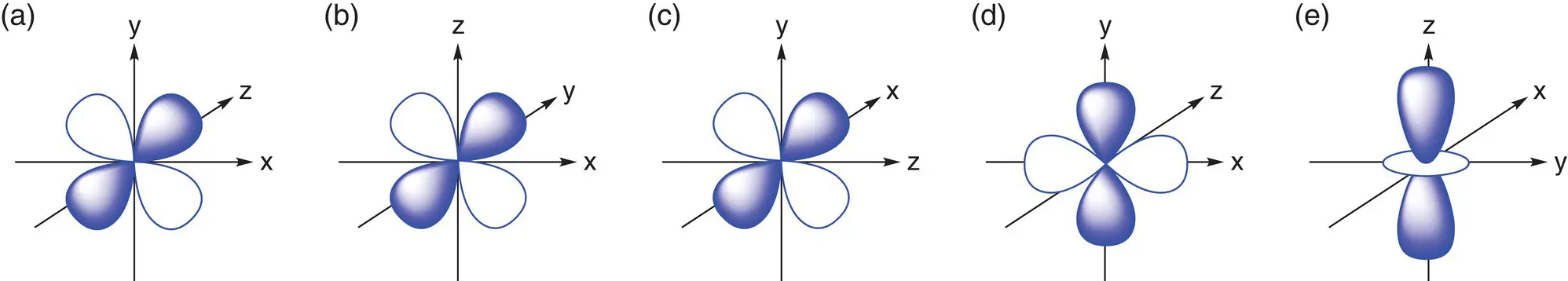
Figure 1.8(a) The d xyorbital; (b) the d xzorbital; (c) the d yzorbital; (d) the d x2–y 2orbital; (e) the d z2orbital.
1.2.5 Describing electronic configurations
Now that you are familiar with the concept of principal quantum levels and atomic orbitals within these levels, the arrangement of electrons in atoms can be seen to follow a logical procedure.
Atomic orbitals are regions in space where there is a high probability of finding an electron. The n = 1 shell has one s orbital. The n = 2 shell has one s orbital and three p orbitals. The n = 3 shell has one s orbital, three p orbitals, and five d orbitals. Each atomic orbital can hold a maximum of two electrons.
As we move further away from the nucleus, the electrons and energy levels have higher energies. Within a principal quantum shell, the electrons in different types of orbitals have different energies. Electrons in s orbitals have a lower energy than electrons in p orbitals, which have a lower energy than electrons in d orbitals. So the order of energies of electrons in atomic orbitals is s < p < d. This is shown in Figure 1.9.
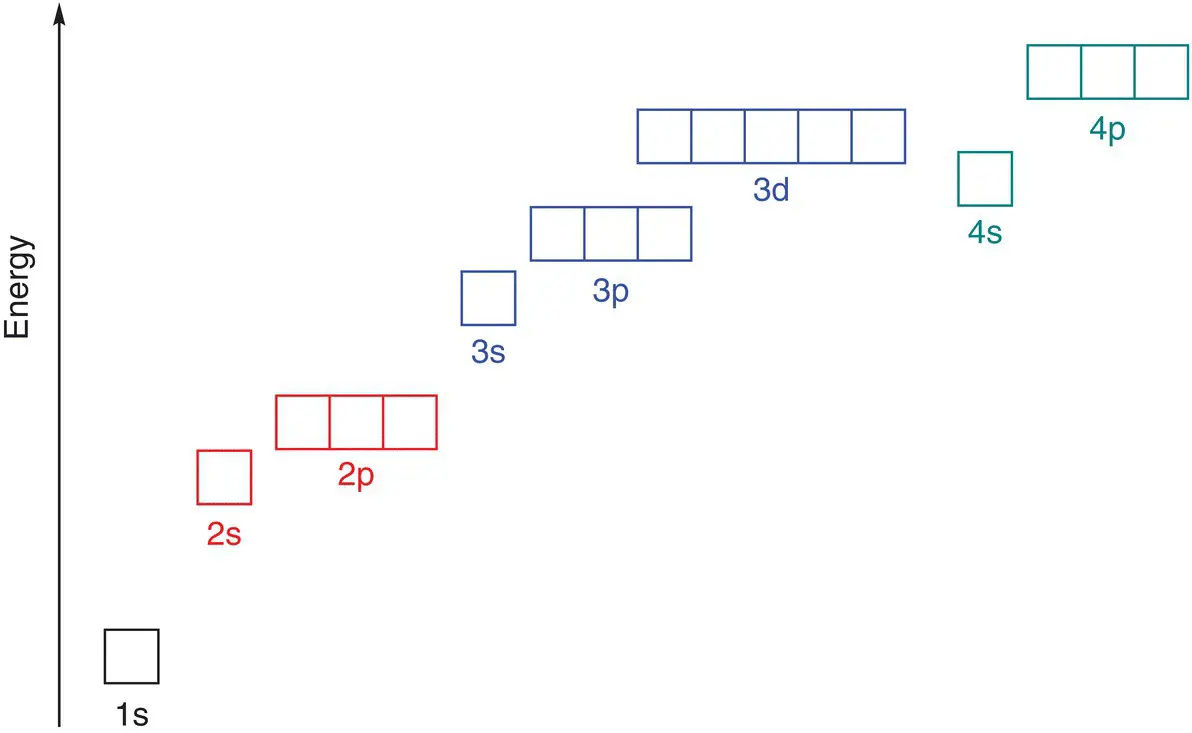
Figure 1.9The relative energies of the orbitals in an atom. Relative energies not to scale.
Electrons fill atomic orbitals according to the following rules:
1 Electrons always go into the lowest energy orbital possible. This is called the Aufbau principle, sometimes known as the building‐up principle.
2 If there is more than one orbital of the same energy available, electrons always fill an unoccupied orbital first: Hund's rule.
3 If two electrons occupy the same orbital, they will have opposite spin: the Pauli exclusion principle.
We have already seen that the electrons for hydrogen and helium obey these rules. The lowest energy orbital is the 1s orbital, and this is filled at helium. The electrons are forced to have opposite spins in helium. The electron configuration for the atom is a shorthand that describes the occupancy of the orbitals. For hydrogen, the electron configuration is 1s 1. For helium, the electron configuration is 1s 2. The superscripted number represents the number of electrons in the 1s orbital for each element.
Once the 1s orbital is full, the next electron at lithium ( Z = 3) must enter the second energy level, as this is the next lowest in energy. Within this energy level, the 2s orbital is of lower energy than the 2p orbitals. Thus the electron occupies the 2s orbital. The electron configuration of lithium is 1s 22s 1.
The next element, beryllium ( Z = 4), has four electrons, and the fourth electron must pair up with the electron in the 2s orbital, as this is of lower energy than the 2p orbitals. The electron configuration is 1s 22s 2. The two electrons in the 2s orbital have opposite spins.
At boron ( Z = 5), the 2s orbital is full, and so the next electron must occupy a 2p orbital. All are empty and of the same energy, and so we arbitrarily place the electron in the 2p xorbital, although it could equally occupy 2p yor 2p z. The electron configuration for boron is 1s 22s 22p 1.
The next two electrons at carbon and nitrogen enter the other empty 2p orbitals. Nitrogen has the electron configuration 1s 22s 22p 3. As all 2p orbitals are half‐filled at nitrogen, the next electron of oxygen must pair with another p electron to give one fully occupied and two half‐occupied 2p orbitals: 1s 22s 22p 4. This can be visualised more easily by using the representation with electrons in boxes, as in Figure 1.10for oxygen.
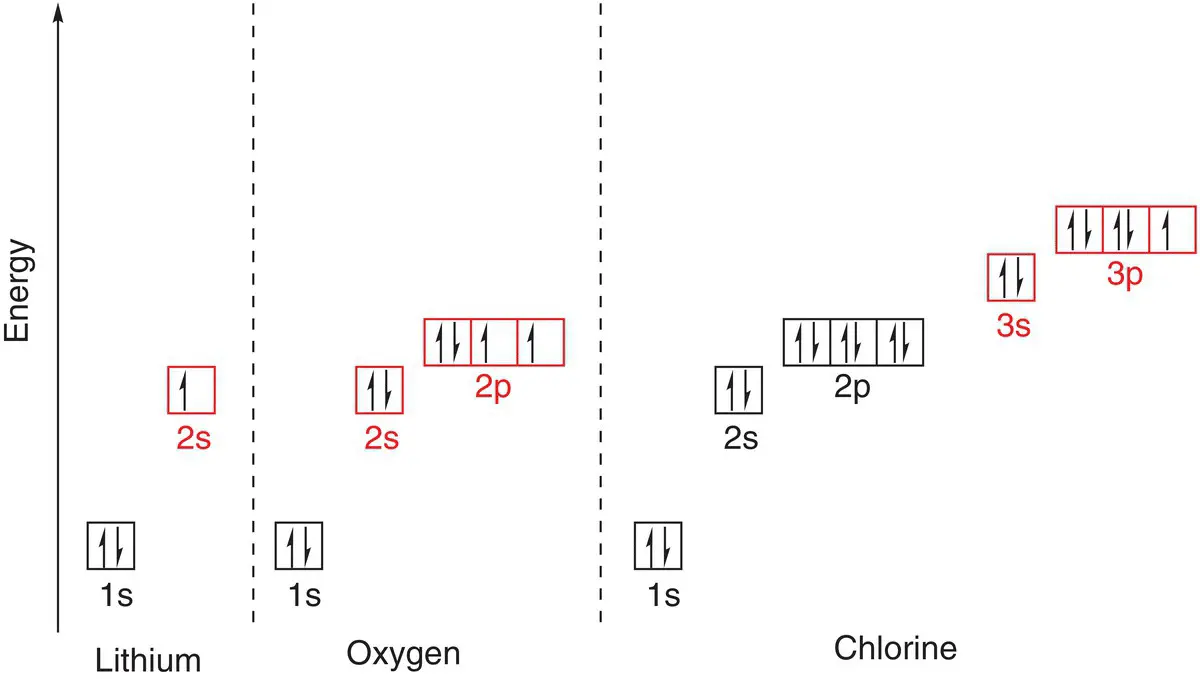
Figure 1.10Electron arrangements in lithium, oxygen, and chlorine. Outer shell electrons are shown in red boxes.
The next electrons complete the remaining two 2p orbitals so that at neon ( Z = 10), we have a filled second shell of electrons: 1s 22s 22p 6. We will see that this is a very stable arrangement of electrons and has significant consequences for the chemical reactivity of the element.
Читать дальше

















