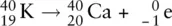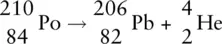
or
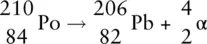
In this case, because 2 protons are emitted from the nucleus, the atomic number of the new element formed is 82, which is the atomic number for lead (Pb). Therefore the element itself changes from polonium to lead. The atomic mass also alters because 2 protons and 2 neutrons have been lost. The new mass number ( A ) is 210 − 4 = 206, which tells us the new element has A = 206.
β −(beta) emission arises when an electron ( 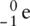 ) is released from the atom and a proton is added to the nucleus. This sounds confusing, as a nucleus does not contain electrons, but what actually happens is that a neutron splits to give a proton and an electron. The proton remains in the nucleus, and the electron is emitted. The atomic number of the atom formed therefore increases by one. β −radiation that generates a proton is usually seen in an element that has a neutron‐rich nucleus. Beta radiation is more penetrating than alpha radiation and can be stopped by a sheet of aluminium foil.
) is released from the atom and a proton is added to the nucleus. This sounds confusing, as a nucleus does not contain electrons, but what actually happens is that a neutron splits to give a proton and an electron. The proton remains in the nucleus, and the electron is emitted. The atomic number of the atom formed therefore increases by one. β −radiation that generates a proton is usually seen in an element that has a neutron‐rich nucleus. Beta radiation is more penetrating than alpha radiation and can be stopped by a sheet of aluminium foil.
An example of beta emission can be seen with the 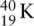 isotope:
isotope:
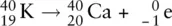
The addition of a proton requires the atomic number to increase by one. In this case, potassium changed to calcium with an atomic number of 20.
Gamma (γ) radiation (or gamma rays) is penetrating electromagnetic radiation released from the nucleus of an atom. It consists of high‐energy photons. Gamma radiation is often released alongside alpha (α) or beta (β) radiation. Gamma radiation is the most strongly penetrating and ionising of the three types of radiation and can only be stopped by using a lead sheet.
Electromagnetic radiation is a continuous range of wavelengths and hence energies of radiation that includes visible light. A photon is the smallest discrete amount, or quantum, of electromagnetic radiation.
This section will outline the structure of the atom in more detail. It describes how electrons are arranged in atoms and how this determines the way the periodic table of the elements is built up.
Figure 1.3shows the arrangement of all 118 known elements in the periodic table. The elements are distributed over 18 columns and 7 rows. The elements are arranged according to atomic number, which increases from left to right and top to bottom. The atomic number equals the number of protons present in a neutral atom and therefore determines the position of the element in the periodic table.
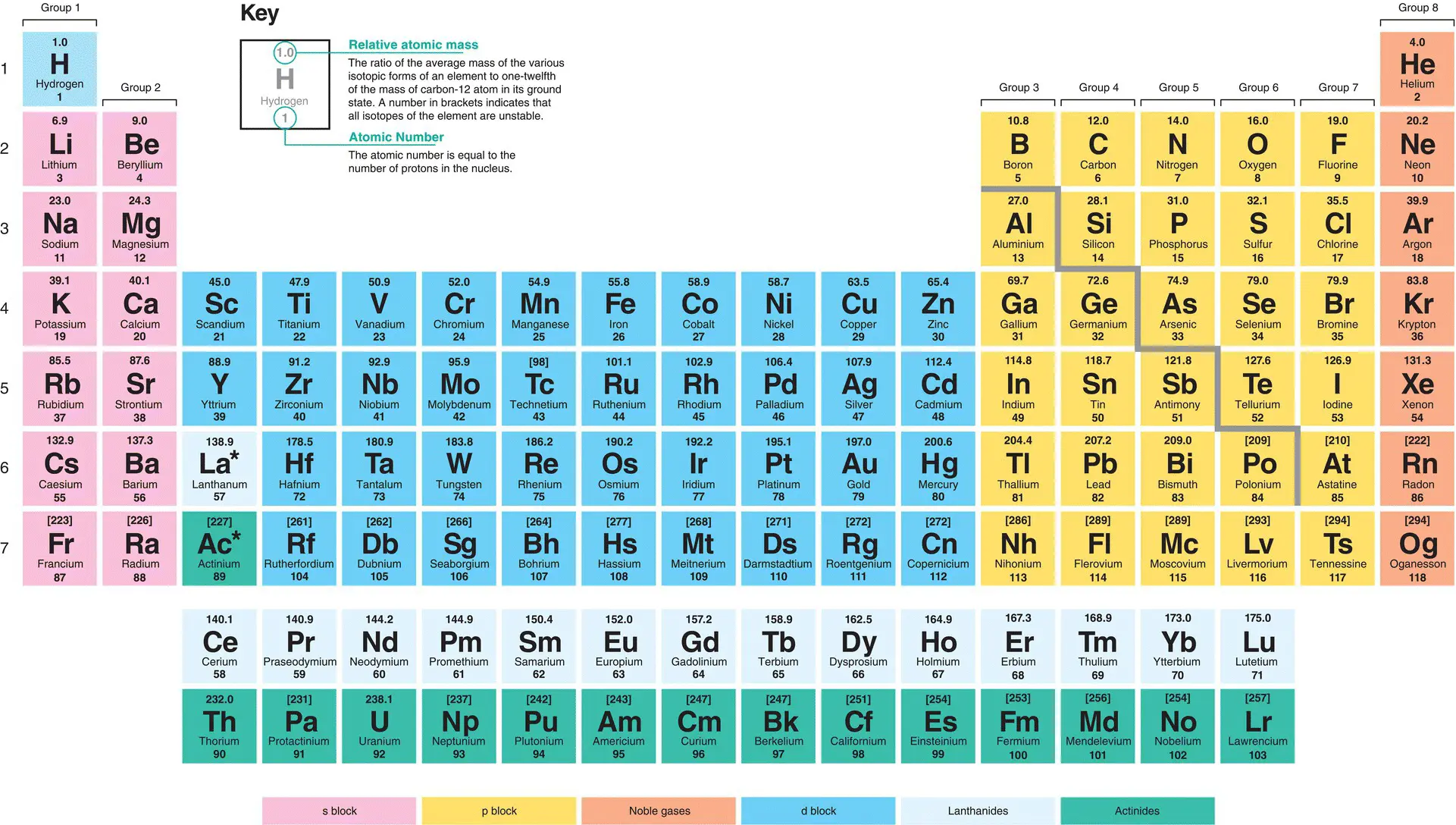
Figure 1.3The standard modern form of the periodic table. Source: University of Reading. Public Domain.
It is best to approach reading the periodic table like a book: start at the top left, and finish at the bottom right.
The rows across the periodic table are called periods, and there are seven periods numbered from one to seven. The columns down the periodic tables are called groups, and there are 18 groups in total, but their naming usually runs from one to eight. All of the elements underneath hydrogen (H) are in Group 1, and beneath beryllium (Be) they are in Group 2. The groups in the middle of the periodic table, from scandium (Sc) to zinc (Zn), are called the d‐block elements and are sometimes referred to by group numbers (i.e. Groups 3 to 12 in the 1-18 numbering system). The elements below boron (B) are in Group 3 (or Group 13), below C are in Group 4 (or Group 14), etc. up to those below helium (He), which are called Group 8 (sometimes called Group 0 or Group 18). Elements that exist in the same group have broadly similar chemical properties, due to having the same number of electrons in their outermost shell.
In some textbooks, you may see Groups 1–8 numbered as Groups 1–18, where the d‐block elements are numbered 3–12.
The periodic table is roughly divided by a zig‐zag line that splits the table into two parts. The elements to the right of the zig‐zag line are non‐metals, and those to the left of the line are metals. There are many more metals than non‐metals in the periodic table. Whether an element is a metal or non‐metal has an impact upon its properties. In addition, the periodic table is split into four distinct areas: the s block, the p block, the d block, and the f block ( Figure 1.3). These areas relate to the orbital in which the outermost electron resides, and will be discussed further in Section 1.2.4.
Metals tend to be lustrous, malleable, sonorous when struck, ductile, and good conductors of heat and electricity. Metals are often solid, with a high melting and boiling point, although the one notable exception is mercury (Hg). Metals usually have a high density and can be heavy. In comparison, non‐metals tend to be dull, brittle, and poor conductors of heat and electricity, and they generally have lower melting and boiling points than metals. Elements that are close to the zig‐zag line exhibit atypical properties and are sometimes referred to as metalloids . For example, silicon (Si) exhibits properties that would be expected for both a metal and a non‐metal.
The bonding properties of metals and non‐metals will be discussed in Chapter 2.
1.2.2 Electron energy levels
Electrons are arranged outside the nucleus of the atom in energy levels that are sometimes called principal quantum shells . You can imagine these as spherical layers extending out from the nucleus as in Figure 1.4. The energy levels are numbered from 1 to 7, with the level closest to the nucleus being level 1. These numbers are referred to as the principal quantum number of the electrons in that energy level. The principal quantum number has the symbol n . As the distance from the nucleus gets bigger, the volume of the layers increases. In addition, as the distance from the nucleus increases, the energy of the level increases along with the energy of the electrons in the level. The seven principal quantum levels relate to the seven rows across the periodic table.
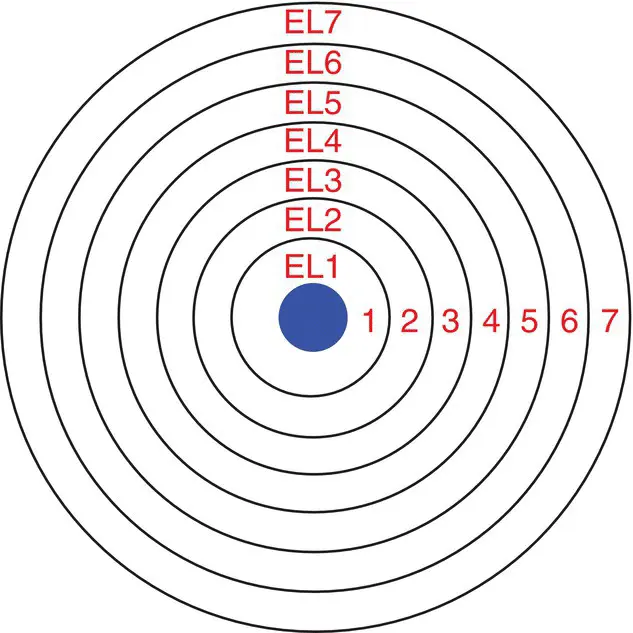
Figure 1.4The energy levels (EL) in an atom. The integers represent the principal quantum number, n .
1.2.3 Simple electronic configurations
The arrangement of electrons in the outer shells or principal quantum levels of the atom is called the electronic configuration . Electrons fill the lowest energy levels in an atom first. As n increases, the energy levels increase in both size and energy and can hold more electrons.
The maximum number of electrons that can be held in the first three energy levels is shown in Table 1.2. In this course, we will not be concerned with electrons in energy levels beyond n = 4.
Читать дальше

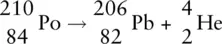
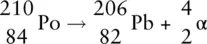
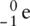 ) is released from the atom and a proton is added to the nucleus. This sounds confusing, as a nucleus does not contain electrons, but what actually happens is that a neutron splits to give a proton and an electron. The proton remains in the nucleus, and the electron is emitted. The atomic number of the atom formed therefore increases by one. β −radiation that generates a proton is usually seen in an element that has a neutron‐rich nucleus. Beta radiation is more penetrating than alpha radiation and can be stopped by a sheet of aluminium foil.
) is released from the atom and a proton is added to the nucleus. This sounds confusing, as a nucleus does not contain electrons, but what actually happens is that a neutron splits to give a proton and an electron. The proton remains in the nucleus, and the electron is emitted. The atomic number of the atom formed therefore increases by one. β −radiation that generates a proton is usually seen in an element that has a neutron‐rich nucleus. Beta radiation is more penetrating than alpha radiation and can be stopped by a sheet of aluminium foil.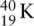 isotope:
isotope: