Table 1.2The maximum number of electrons in the first four energy levels.
| Energy level or shell |
n |
Maximum number of electrons |
| First |
1 |
2 |
| Second |
2 |
8 |
| Third |
3 |
18 |
| Fourth |
4 |
32 |
The first shell can contain only two electrons. The elements that only have electrons in the first shell are hydrogen and helium, with one and two electrons, respectively. Once the first shell is full, at helium, the next electron (in lithium) must be placed in the second shell. The second shell can hold up to eight electrons. Once a shell is full, an element is said to be stable . The noble gases (Group 8, also called Group 18) all have a full outer shell, so they are stable and are very unreactive. Table 1.3shows the arrangement of the electrons in shells for the first 11 elements in the periodic table.
Table 1.3Arrangement of electrons in the first 11 elements of the periodic table.
| Element |
Number of electrons or Z |
Number in 1st shell ( n = 1) |
Number in 2nd shell ( n = 2) |
Number in 3rd shell ( n = 3) |
| H |
1 |
1 |
0 |
0 |
| He |
2 |
2 |
0 |
0 |
| Li |
3 |
2 |
1 |
0 |
| Be |
4 |
2 |
2 |
0 |
| B |
5 |
2 |
3 |
0 |
| C |
6 |
2 |
4 |
0 |
| N |
7 |
2 |
5 |
0 |
| O |
8 |
2 |
6 |
0 |
| F |
9 |
2 |
7 |
0 |
| Ne |
10 |
2 |
8 |
0 |
| Na |
11 |
2 |
8 |
1 |
You can see from the table that each consecutive element has one more electron, and these fill energy levels from n = 1 upwards. The first energy level is full at helium, and so the next electron (in lithium) enters the n = 2 shell. The second energy level is full at neon ( Z = 10), and so the next electron (in sodium) enters the n = 3 energy level.
Electron configurations can be represented as in the diagram in Figure 1.5for neon. This shows that the first and second shells are both full.
You will notice that there are four paired electrons and two single electrons in the second energy level of the O atom in Worked Example 1.4. Electrons in the same energy level tend to remain unpaired unless they have to pair up with another electron.
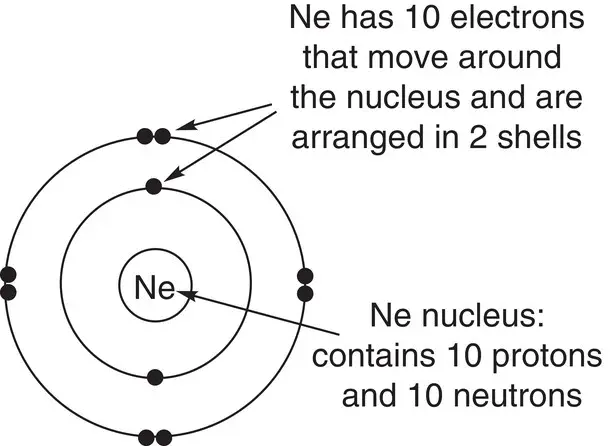
Figure 1.5The structure of neon.
Draw the arrangement of electrons in oxygen.
The position of oxygen in the periodic table and its atomic number ( Z = 8) tell us that a neutral atom of oxygen has 8 protons and therefore must have 8 electrons. However, oxygen is in the second row of the periodic table; therefore the electrons are arranged into two shells. The first shell contains two electrons because there is only space for two electrons, and the second shell contains six electrons. We can draw this as shown:
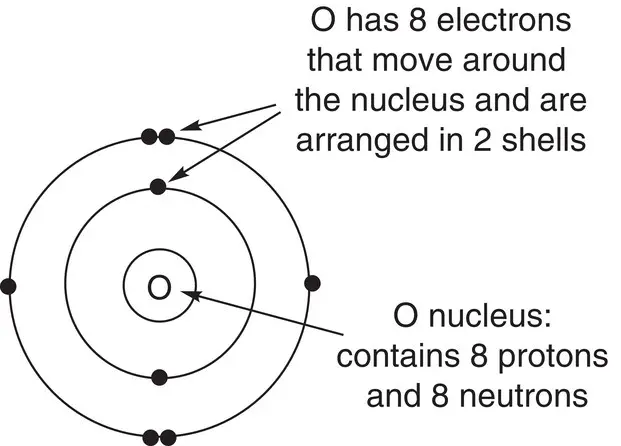
We have seen that each successive shell in an atom can hold an increasing number of electrons. The reason for the precise maximum number of electrons that each shell can hold is due to the arrangement of the electrons in each shell. The next section will explain how electrons are arranged in shells or energy levels.
1.2.4 Sub‐shells and atomic orbitals
Within each energy level, there are distinct areas in space that the electrons occupy. These distinct areas are sub‐shells , which are further arranged into atomic orbitals . A sub‐shell is a group of atomic orbitals that have the same properties, such as shape. The sub‐shells are called s, p, d, and f. Atomic orbitals are regions within a sub-shell that can contain up to two electrons and have a particular orientation in space. For example, the p sub‐shell actually comprises three atomic orbitals, which are called p‐orbitals. To understand about atomic orbitals and sub‐shells, it is useful to spend a little time considering the nature of electrons.
We have seen that electrons are very small, they have a negative charge, and they move very quickly. The early twentieth century was an exciting time for physicists and chemists. Many ground‐breaking experiments were carried out that helped determine the properties of the three main fundamental atomic particles: the proton, the neutron, and the electron. Unfortunately, there isn't time or space to describe these experiments here, but two important experiments gave scientists unexpected and potentially conflicting information about the properties of electrons. These experiments showed that electrons can behave as both particles and waves, and this theory became known as wave‐particle duality . A consequence of wave‐particle duality is Heisenberg's Uncertainty Principle, which states that it is impossible to accurately know both the position and velocity of an electron at the same time. Around the same time, a physicist named Schrödinger produced a mathematical model of the atom, which allowed for the wave‐like behaviour of electrons and produced equations that described the position of an electron in the outer shells of an atom. The equations that Schrödinger derived led to the idea that there are regions in space around the nucleus where there is a high probability of finding an electron of a given energy. These regions in space are called atomic orbitals and can be thought of as sub‐shells within the principal quantum shells.
When considering the occupancy of electrons in atomic orbitals or the behaviour of electrons in chemical reactions, it is often more intuitive to think of electrons as small negatively charged particles – and this is fine. However, it is important to remember that electrons also have wave‐like properties, which prevents us from stating exactly where the electron is at any one time; we can only say where there is a good probability of finding the electron.
As stated earlier electrons in each principal quantum shell of an atom are arranged in orbitals. An orbital is an area in space where there is a high probability of finding an electron of a specific energy. An orbital is actually a mathematical function that describes the wave‐like behaviour of an electron in an atom. When this function is plotted in three dimensions for an electron of a certain energy, a three‐dimensional shape is obtained. The shape represents the space in which there is a high probability of finding an electron with that energy. An atomic orbital can hold a maximum of two electrons.
Any atomic orbital can hold a maximum of two electrons.
The hydrogen atom has just one electron, which occupies the first principal quantum shell. There is just one atomic orbital in this shell, called a 1s orbital. The number 1 tells us the orbital is in the first principal quantum shell ( n = 1).
An s orbital is spherical in shape, and in any shell, there is only one s orbital. A 1s orbital is shown in Figure 1.6a. Also shown in this figure are a 2s orbital and a 3s orbital ( Figures 1.6b and c). They are all spherical in shape and get larger as the value of n increases. Within an atom, all the s orbitals are concentric – this means they all have the nucleus as their centre and lie on top of each other. The shading in the figure represents the electron density or the probability of finding an electron in each orbital. You can see that in the 1s orbital, there is a high probability of finding the electron close to the nucleus. However, in the higher s orbitals, there are areas that are unshaded and therefore are unlikely to contain electrons. These areas are called nodes .
Читать дальше














