Each element is described by the number of protons, neutrons, and electrons it possesses. These are represented by two quantities: the mass number ( A ) and the atomic number ( Z ) The mass number indicates the total number of protons and neutrons in the nucleus of the atom (p + n). The atomic number gives the number of protons in a neutral atom of the element (p). A neutral atom must have the same number of protons as electrons, so Z also indicates the number of electrons (e).
The mass number gives the total number of protons and neutrons: A = p + n. The atomic number gives the total number of protons or electrons present in a neutral atom: Z = p or e.
For any element X, we can represent the information about the mass number, A , and atomic number, Z , as shown in Figure 1.2a. As you can see, the mass number is written as a superscript and the atomic number as a subscript in front of the symbol for the element.
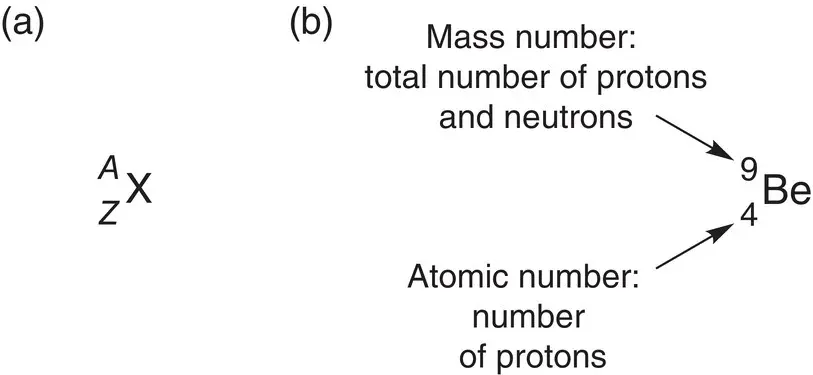
Figure 1.2(a) General representation of mass number and atomic number for the element X. (b) The mass number and atomic number for beryllium.
Information about the element beryllium is shown in Figure 1.2b using this convention.
Beryllium (Be) has an atomic number, Z , of 4; therefore, in a neutral molecule, there are 4 protons and 4 electrons. Beryllium has a mass number, A , of 9; therefore it has 5 neutrons (because the mass number is 9 and we already know that it has 4 protons from the atomic number, so 9 − 4 = 5 neutrons). The mass number is written as a superscript and the atomic number as a subscript before the symbol for the element.
How many protons, electrons, and neutrons are present in an atom of aluminium, 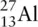 ?
?
Aluminium exists as 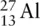 . The mass number is 27, and the atomic number is 13. The atomic number tells us how many protons there are, so in a neutral atom of aluminium, there are 13 protons, and there must be 13 electrons to balance the charge from the protons. If there are 13 protons, there must be 14 neutrons, because the number of protons and neutrons adds up to the mass number, 27.
. The mass number is 27, and the atomic number is 13. The atomic number tells us how many protons there are, so in a neutral atom of aluminium, there are 13 protons, and there must be 13 electrons to balance the charge from the protons. If there are 13 protons, there must be 14 neutrons, because the number of protons and neutrons adds up to the mass number, 27.
How many protons, electrons, and neutrons are present in an oxide (O 2−) ion? The symbol for an oxygen atom is 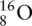 .
.
Oxygen exists as 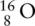 . An oxide ion is an oxygen atom with a 2− charge, so it has 2 extra electrons compared to a neutral oxygen atom. Therefore: oxygen contains 8 protons (due to the mass number) and 8 neutrons (due to the difference between the mass number and the atomic number); and, because it has a 2− charge, it has to contain 2 more electrons than it has protons (because each electron has a negative charge), so it has 10 electrons.
. An oxide ion is an oxygen atom with a 2− charge, so it has 2 extra electrons compared to a neutral oxygen atom. Therefore: oxygen contains 8 protons (due to the mass number) and 8 neutrons (due to the difference between the mass number and the atomic number); and, because it has a 2− charge, it has to contain 2 more electrons than it has protons (because each electron has a negative charge), so it has 10 electrons.
Remember from the previous chapter that an ion is an atom or a group of atoms that has a charge.
Many elements possess isotopes. An isotope is an atom of an element that has a different mass number. Because all isotopes of the same element have the same atomic number ( Z ), the number of protons must be the same. This is always the case. If the number of protons has changed, the element has also changed! Isotopes differ in the number of neutrons in the nucleus of the atom. An example of an element that has isotopes is bromine, which naturally exists in two forms:
contains 35 protons, 35 electrons, and 44 neutrons.
contains 35 protons, 35 electrons, and 46 neutrons.
These isotopes are sometimes written as bromine‐79 ( 79Br) and bromine‐81 ( 81Br).
You will meet the average relative atomic mass of an element in Chapter 3.1.1.
An isotope is an atom of an element that has the same atomic number but differs in the number of neutrons and therefore mass number.
In naturally occurring bromine, the ratio of 79Br to 81Br is 50.5 : 49.5. This means that in 100 bromine atoms, 50.5 will be 79Br and 49.5 will be 81Br. The proportion naturally occurring of each isotope is called the relative abundanceor isotopic abundance. Knowing the relative abundance of each isotope and its mass number allows us to calculate the average mass of one atom of the element as shown here for bromine:
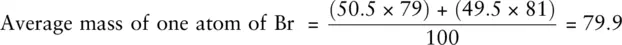
Note that the answer calculated has been given no units as A , the mass number, is unitless. The actual unit for the answer is the atomic mass unit (amu), which is an extremely small quantity. However, the unitless answer obtained is equal to the average relative atomic mass, Ar , of the element which is covered in Chapter 3.
Uranium exists as three isotopes: 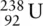 ,
, 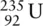 , and
, and 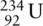 with approximate relative abundance 99.27%, 0.72%, and 0.01%, respectively. What is the average relative atomic mass of a uranium atom to three significant figures?
with approximate relative abundance 99.27%, 0.72%, and 0.01%, respectively. What is the average relative atomic mass of a uranium atom to three significant figures?
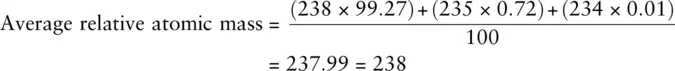
Some isotopes are unstable and emit radiation; they are radioactive. Three types of emission are produced spontaneously from radioactive nuclei: alpha (α), beta (β), and gamma (γ). The exact species that is emitted is dependent upon the type of emission.
An alpha particle, α, has two protons and two neutrons – it is effectively the nucleus of a He atom. Compared to a helium atom, the alpha particle, α, has lost both of its electrons and therefore has a 2+ charge. We can represent it as: 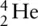 or
or 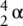 . Alpha (α) emission occurs when an alpha particle or helium nucleus is lost from the radioisotope. This is usually restricted to elements with atomic numbers over 83 that have a large proton‐to‐neutron ratio. This emission causes a change in the composition of the nucleus of the element from which it was emitted, generating a new element. Alpha radiation is not very penetrating and can be stopped by either paper or skin.
. Alpha (α) emission occurs when an alpha particle or helium nucleus is lost from the radioisotope. This is usually restricted to elements with atomic numbers over 83 that have a large proton‐to‐neutron ratio. This emission causes a change in the composition of the nucleus of the element from which it was emitted, generating a new element. Alpha radiation is not very penetrating and can be stopped by either paper or skin.
Alpha (α) particles are emitted by 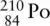 . An equation can be written for this radioactive decay process:
. An equation can be written for this radioactive decay process:
Читать дальше


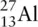 ?
?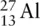 . The mass number is 27, and the atomic number is 13. The atomic number tells us how many protons there are, so in a neutral atom of aluminium, there are 13 protons, and there must be 13 electrons to balance the charge from the protons. If there are 13 protons, there must be 14 neutrons, because the number of protons and neutrons adds up to the mass number, 27.
. The mass number is 27, and the atomic number is 13. The atomic number tells us how many protons there are, so in a neutral atom of aluminium, there are 13 protons, and there must be 13 electrons to balance the charge from the protons. If there are 13 protons, there must be 14 neutrons, because the number of protons and neutrons adds up to the mass number, 27.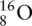 .
.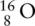 . An oxide ion is an oxygen atom with a 2− charge, so it has 2 extra electrons compared to a neutral oxygen atom. Therefore: oxygen contains 8 protons (due to the mass number) and 8 neutrons (due to the difference between the mass number and the atomic number); and, because it has a 2− charge, it has to contain 2 more electrons than it has protons (because each electron has a negative charge), so it has 10 electrons.
. An oxide ion is an oxygen atom with a 2− charge, so it has 2 extra electrons compared to a neutral oxygen atom. Therefore: oxygen contains 8 protons (due to the mass number) and 8 neutrons (due to the difference between the mass number and the atomic number); and, because it has a 2− charge, it has to contain 2 more electrons than it has protons (because each electron has a negative charge), so it has 10 electrons.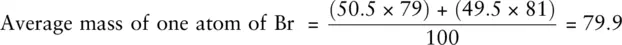
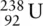 ,
, 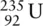 , and
, and 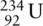 with approximate relative abundance 99.27%, 0.72%, and 0.01%, respectively. What is the average relative atomic mass of a uranium atom to three significant figures?
with approximate relative abundance 99.27%, 0.72%, and 0.01%, respectively. What is the average relative atomic mass of a uranium atom to three significant figures?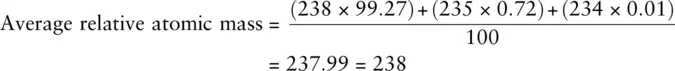
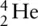 or
or 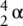 . Alpha (α) emission occurs when an alpha particle or helium nucleus is lost from the radioisotope. This is usually restricted to elements with atomic numbers over 83 that have a large proton‐to‐neutron ratio. This emission causes a change in the composition of the nucleus of the element from which it was emitted, generating a new element. Alpha radiation is not very penetrating and can be stopped by either paper or skin.
. Alpha (α) emission occurs when an alpha particle or helium nucleus is lost from the radioisotope. This is usually restricted to elements with atomic numbers over 83 that have a large proton‐to‐neutron ratio. This emission causes a change in the composition of the nucleus of the element from which it was emitted, generating a new element. Alpha radiation is not very penetrating and can be stopped by either paper or skin.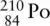 . An equation can be written for this radioactive decay process:
. An equation can be written for this radioactive decay process:










