Give the ground state electron configuration for 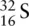 .
.
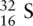 has atomic number 16 and mass number 32. Therefore, a neutral atom of S has 16 protons, 16 electrons, and 16 neutrons. When putting the electrons into the orbitals, it is important to always start with the lowest‐energy orbital.
has atomic number 16 and mass number 32. Therefore, a neutral atom of S has 16 protons, 16 electrons, and 16 neutrons. When putting the electrons into the orbitals, it is important to always start with the lowest‐energy orbital.
Electrons are placed in the energy levels starting at the lowest energy level (1s) in order of increasing energy (Aufbau principle). When filling the third shell, electrons are firstly placed in the 3s orbital, as this has the lowest energy in the third shell, followed by four electrons in the 3p orbitals. However, when putting the remaining four electrons in the 3p orbitals, each p orbital is filled with one electron first (Hund's rule). When each p orbital is half full, there is one electron left over, and it is at this point that the electrons are paired. When they are paired, they have opposing spins, which in this case is represented by drawing each half‐arrow in opposite directions, fulfilling the requirements of the Pauli exclusion principle.
The ground state electronic configuration of sulfur can be written as 1s 22s 22p 63s 23p 4.
The reason we say ‘ground state’ electron configuration is because ‘excited states’ can also exist. The discussion of excited states is beyond the scope of this book but is discussed at degree level.
Give the ground state electron configuration for  .
.
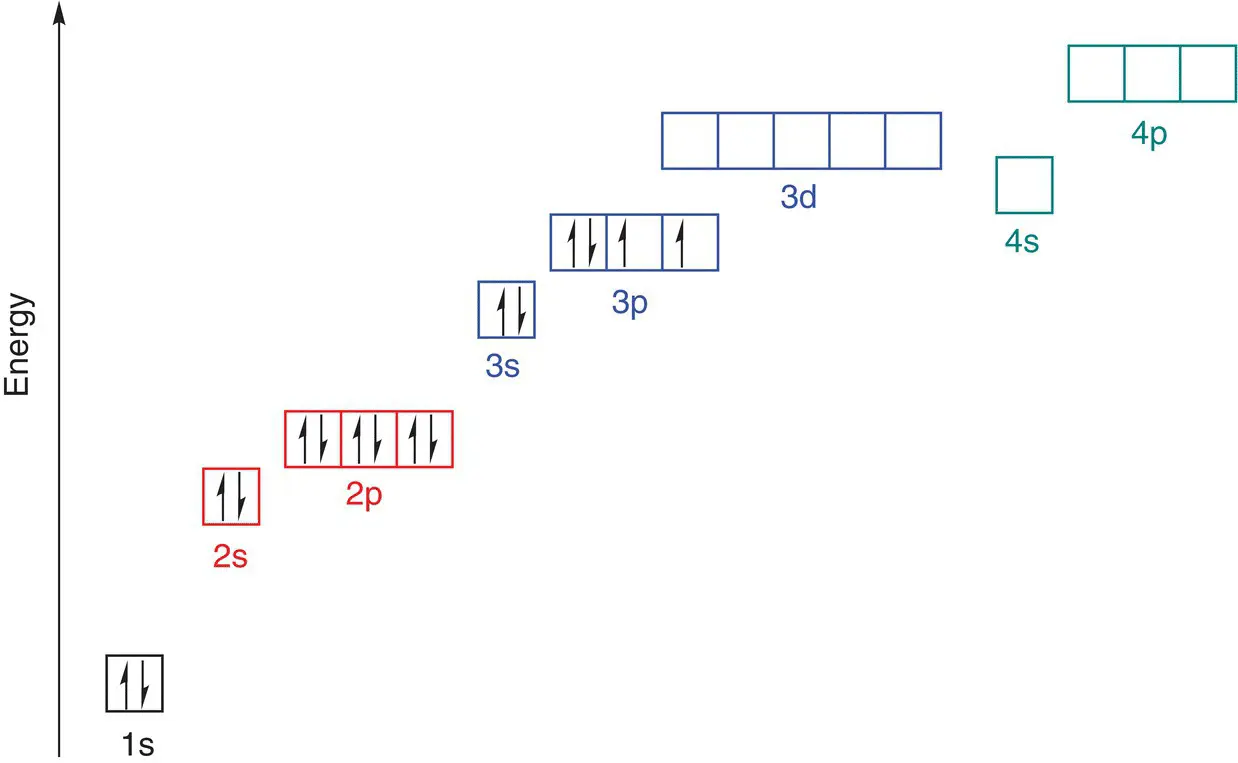
 has atomic number 19 and mass number 39, therefore in a neutral atom there are 19 protons, 19 electrons, and 20 neutrons.
has atomic number 19 and mass number 39, therefore in a neutral atom there are 19 protons, 19 electrons, and 20 neutrons.

Starting at the lowest energy level, electrons are placed in the energy levels in order of increasing energy. When the third shell is reached, the electrons are firstly placed in the 3s orbital, as this is the lowest energy in the third shell, followed by six electrons in the 3p orbitals. There is one electron left over, and this is put into the 4s orbital because this is lower in energy than the 3d orbitals. This explains why potassium is an s‐block element, as the highest energy electron is in an s orbital.
The ground state electronic configuration of potassium can be written as 1s 22s 22p 63s 23p 64s 1or [Ar] 4s 1.
Give the ground state electron configuration for scandium.
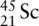 has atomic number 21 and mass number 45. Therefore, in a neutral atom of Sc, there are 21 protons, 21 electrons, and 24 neutrons. As with the other examples, and following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, the ground state electronic configuration of scandium can be written as 1s 22s 22p 63s 23p 64s 23d 1or [Ar]4s 23d 1. Remember, the 4s orbital is filled before the 3d because it is lower in energy.
has atomic number 21 and mass number 45. Therefore, in a neutral atom of Sc, there are 21 protons, 21 electrons, and 24 neutrons. As with the other examples, and following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, the ground state electronic configuration of scandium can be written as 1s 22s 22p 63s 23p 64s 23d 1or [Ar]4s 23d 1. Remember, the 4s orbital is filled before the 3d because it is lower in energy.

Atoms are made up of a central, dense region containing neutrons and protons surrounded by electrons in outer shells with different energies.
Protons and neutrons have the same relative masses as each other whereas electrons are almost 2000 times lighter. Protons have a positive charge, electrons have a negative charge and neutrons are neutral. Isotopes of an element have the same number of protons (Z) but different numbers of neutrons and therefore different mass number (A).
There are three main types of radioactive decay namely alpha-, beta- and gamma-.
Electrons are arranged in orbitals according to their energies in the outer shells, or energy levels of the atom.
The ground state electronic configuration of an atom can be defined by using the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
Each successive element has one more proton in the nucleus and one more electron in its outer shell. The different areas of the periodic table (s block, p block etc) are characterised by the type of orbitals in the outer shells being filled by electrons.
1 What are the relative mass and charge of a proton, a neutron, and an electron?
2 How many protons, neutrons, and electrons do the following atoms and ions contain?
3 Define the term isotope. Include an example in your answer.
4 Zinc exists as five naturally occurring isotopes: 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn, with abundances 49.2%, 27.7%, 4.0%, 18.5%, and 0.6%, respectively. Calculate the average relative atomic mass of zinc.
5 What are the three types of radiation that can be emitted by radioisotopes? Explain the difference between each type, and give any relevant equations.
6 222Rn undergoes alpha decay. Give the equation for this process.
7 Draw a clearly annotated diagram showing the shells of electrons in the following elements:ChlorineCalciumNeonCarbon
8 On a clearly annotated diagram, draw and label:A 1s orbitalThree 2p orbitalsFive 3d orbitals
9 Write the ground state electronic configurations for the following atoms or ions:SSrSe2−CoMnMg2+
10 State the elements that have the following ground state electronic configurations:1s22s22p21s22s22p63s23p31s22s22p63s23p63d104s24p6[Ar]3d54s1
At the end of this chapter, students should be able to:
Describe different types of bonding using dot‐and‐cross diagrams where appropriate, namely:Simple molecular and giant covalent bondingIonic bondingMetallic bonding
Deduce and explain the shapes of simple molecules
Explain polarity and intermolecular forces
Identify dipoles
Deduce the strength of intermolecular forces and therefore some properties of a material
This section will outline the type of bonding that exists in covalent molecules. It will also describe ionic bonding and metallic bonding.
2.1.1 Atoms and molecules
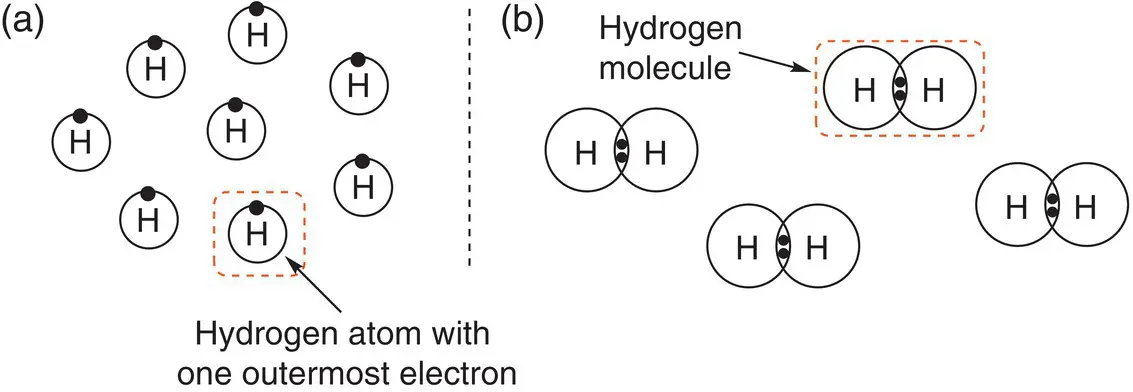
In the first chapter, we saw that an atomis an individual entity that contains electrons, protons, and neutrons. A moleculeis formed when two (or more) atoms bond together. Some elements exist naturally as molecules, such as chlorine, Cl 2, nitrogen, N 2, and hydrogen, H 2. For example, Figure 2.1, shows eight hydrogen atoms and the four hydrogen molecules they form by bonding together.
Читать дальше

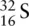 .
.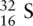 has atomic number 16 and mass number 32. Therefore, a neutral atom of S has 16 protons, 16 electrons, and 16 neutrons. When putting the electrons into the orbitals, it is important to always start with the lowest‐energy orbital.
has atomic number 16 and mass number 32. Therefore, a neutral atom of S has 16 protons, 16 electrons, and 16 neutrons. When putting the electrons into the orbitals, it is important to always start with the lowest‐energy orbital.
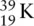 .
. has atomic number 19 and mass number 39, therefore in a neutral atom there are 19 protons, 19 electrons, and 20 neutrons.
has atomic number 19 and mass number 39, therefore in a neutral atom there are 19 protons, 19 electrons, and 20 neutrons.
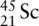 has atomic number 21 and mass number 45. Therefore, in a neutral atom of Sc, there are 21 protons, 21 electrons, and 24 neutrons. As with the other examples, and following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, the ground state electronic configuration of scandium can be written as 1s 22s 22p 63s 23p 64s 23d 1or [Ar]4s 23d 1. Remember, the 4s orbital is filled before the 3d because it is lower in energy.
has atomic number 21 and mass number 45. Therefore, in a neutral atom of Sc, there are 21 protons, 21 electrons, and 24 neutrons. As with the other examples, and following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, the ground state electronic configuration of scandium can be written as 1s 22s 22p 63s 23p 64s 23d 1or [Ar]4s 23d 1. Remember, the 4s orbital is filled before the 3d because it is lower in energy.












