Anti‐Infective Medicines/Antibacterials/Beta‐Lactam Medicines
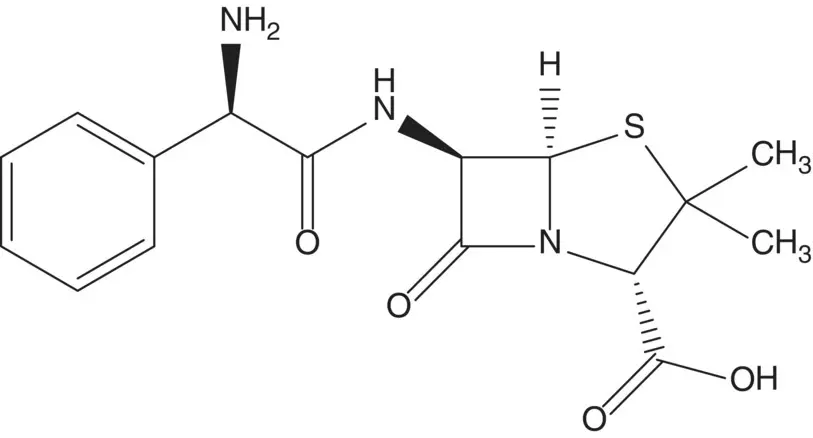
Penicillins are produced by fermentation or are semisynthetic. A semisynthetic penicillin is often formed by acylation of the amine of 6‐aminopenicillanic acid (6‐APA). 6‐APA is produced from penicillin G (benzylpenicillin) by enzyme‐mediated hydrolysis of the side‐chain amide.
Discussion.Ampicillin is a semisynthetic penicillin. The final step is release of the amine by hydrolysis of an enamine. The side‐chain amide is formed by enzyme‐mediated formation of the reaction of 6‐aminopenicillanic acid (6‐APA) with a mixed anhydride. 6‐Aminopenicillanic acid is formed by enzyme‐mediated hydrolysis of the side‐chain amide of penicillin G. Penicillin G (benzylpenicillin) is produced by the fungus P. chrysogenum .
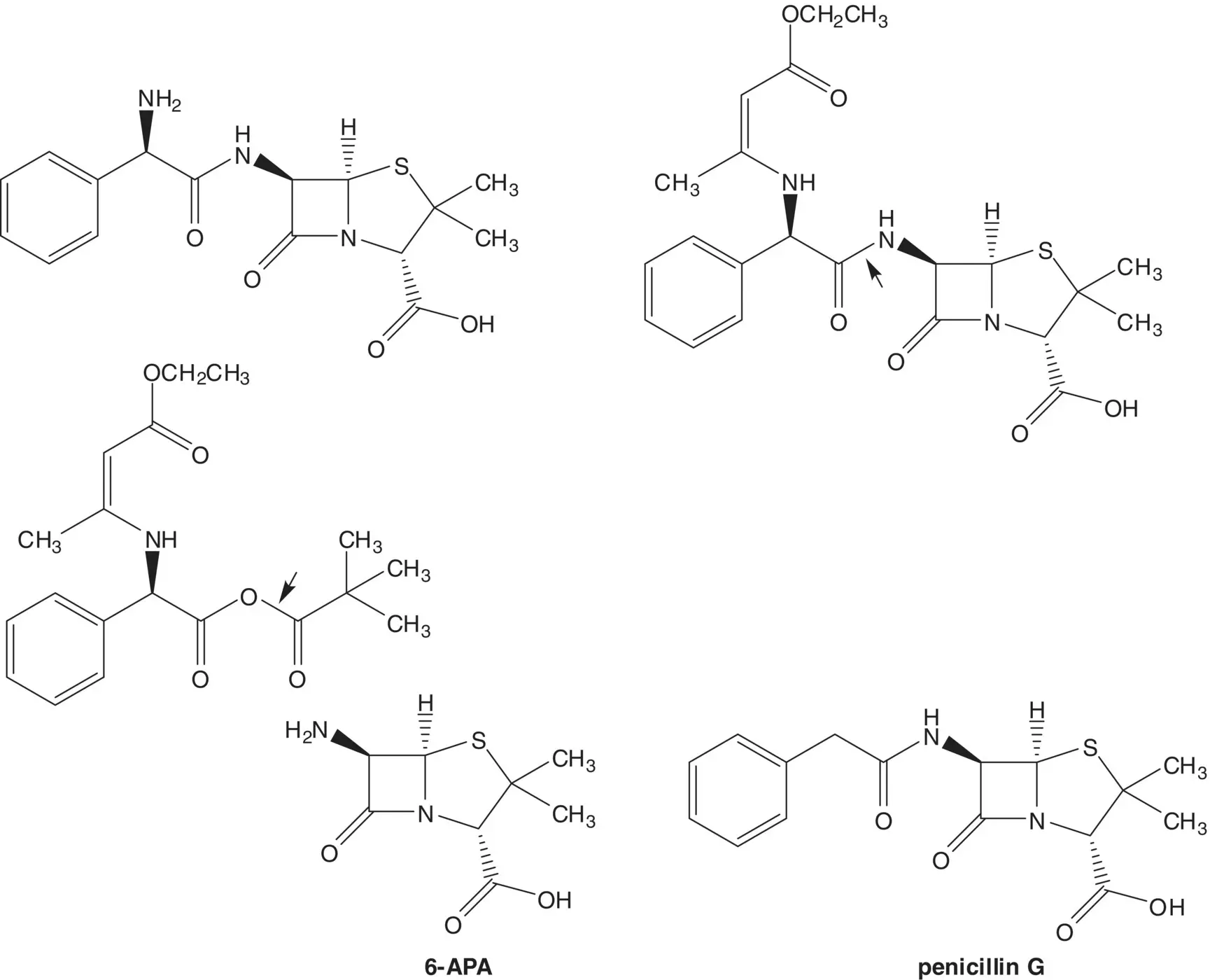
The mixed anhydride is formed from the potassium carboxylate salt and pivaloyl chloride. The N‐protected potassium carboxylate salt of the amino acid (known as a Dane Salt) is formed from ( R )‐α‐phenylglycine and ethyl acetoacetate.
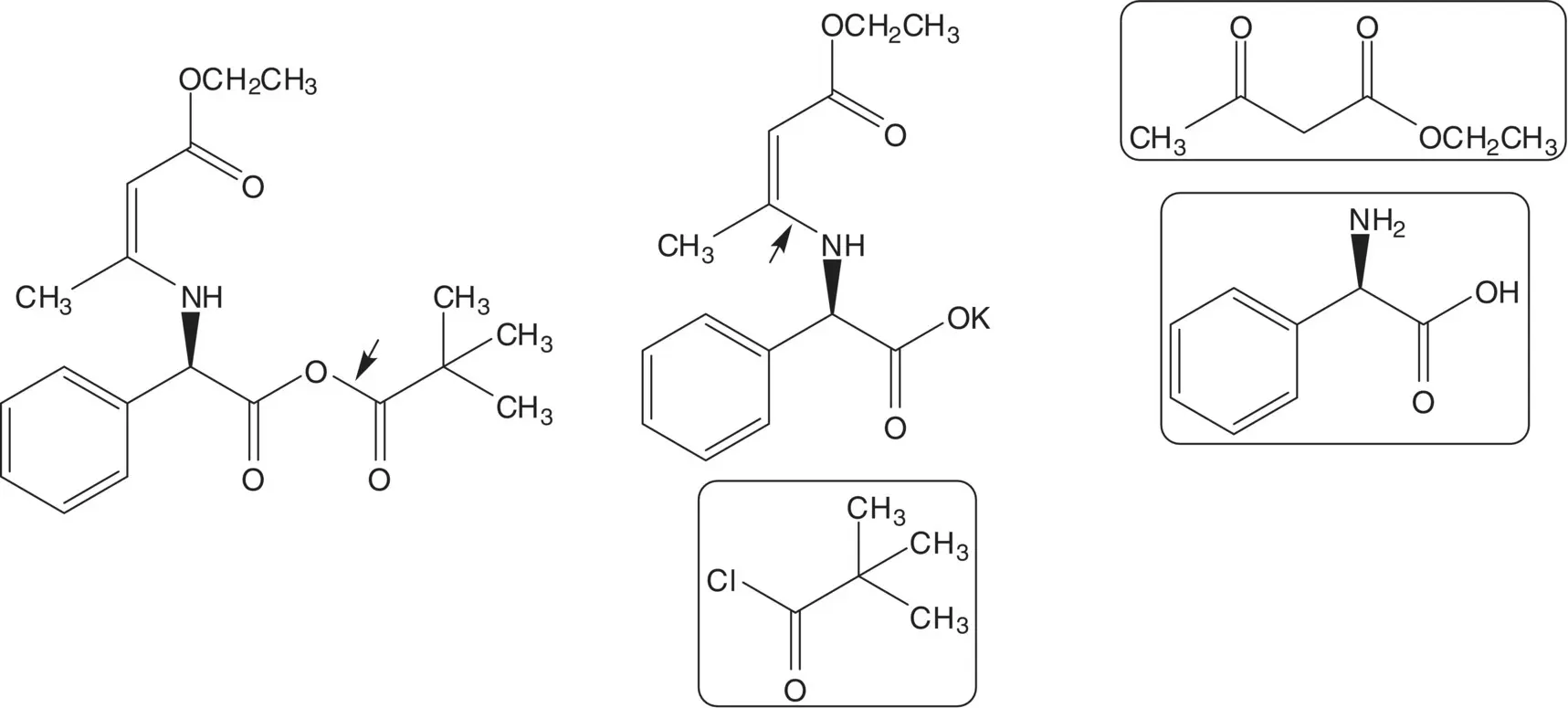
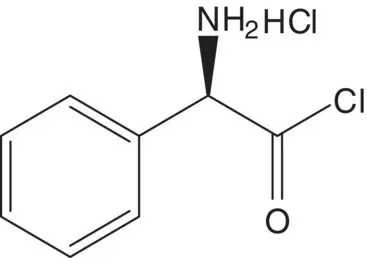
Ampicillin is also manufactured from 6‐APA and the acid chloride, ( R )‐α‐phenylglycine chloride hydrochloride. List the pros and cons for both routes and select one route as the preferred route.
Antineoplastics and Immunosuppressives/Hormones and Antihormones
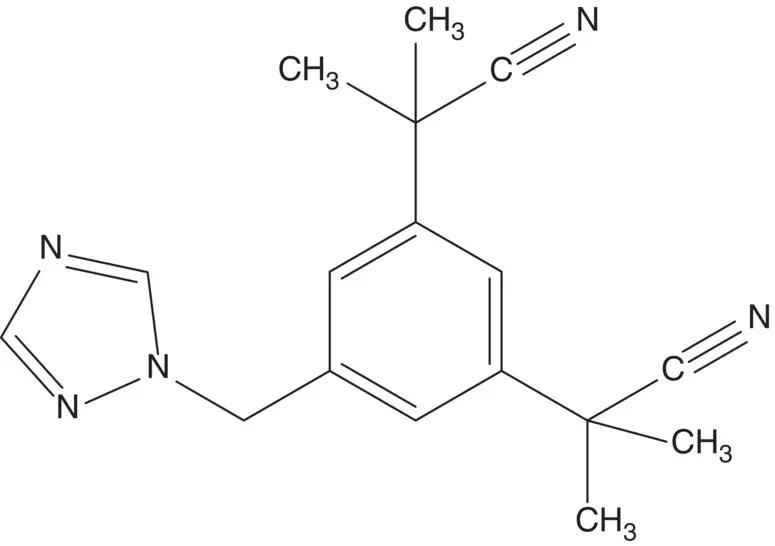
A phenylacetonitrile is often formed by displacement of a benzyl chloride or bromide by cyanide.
Discussion.Each substituent on the central ring of anastrozole has a functional group (cyanide or 1,2,4‐triazole) which is likely introduced as a nucleophile. These features suggest disconnection strategies which have statistical product distribution problems. In a preferred strategy, the most significant problem is addressed in the preparation of the starting material.
Bromide is displaced by 1,2,4‐triazole in the final step. The bromomethyl group is formed by bromination of the methyl group.
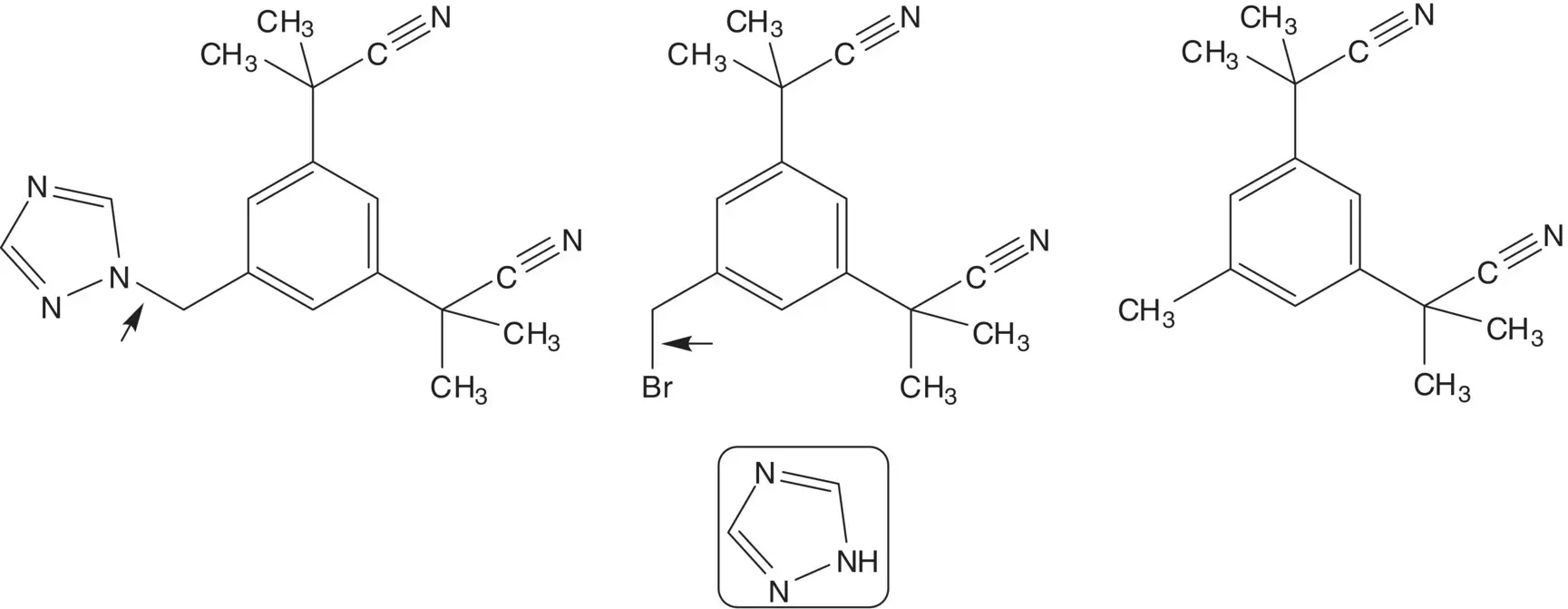
Four methyl groups are added by α‐alkylation of the nitriles with iodomethane. The nitriles are formed by bromide displacement by sodium cyanide. The dibromide is formed by bromination of mesitylene.
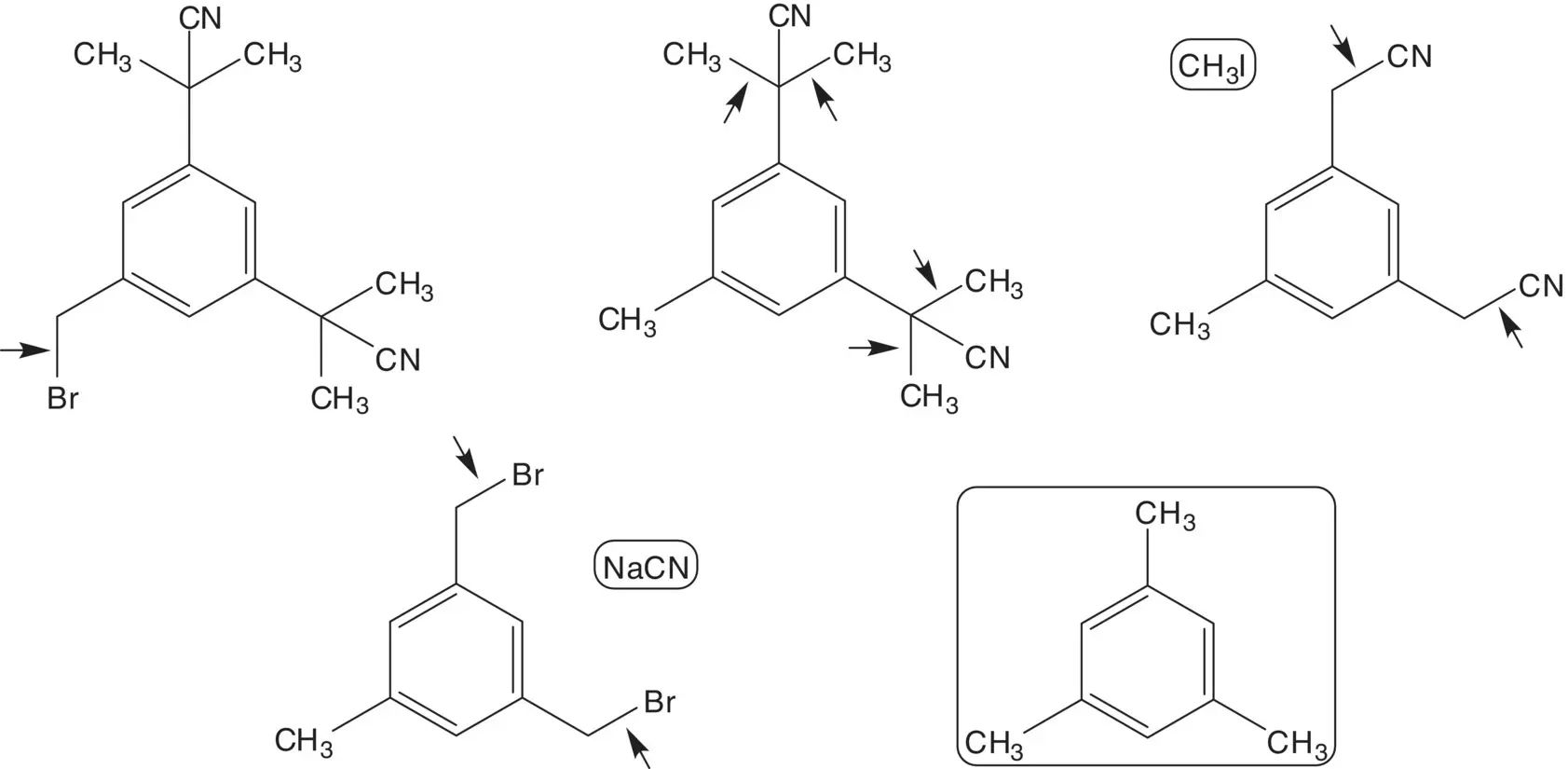
Final product purity is critical when manufacturing a drug substance. To ensure high purity of the product, no side products which are difficult to separate from the product should form in the final step. This is not the case for the anastrozole process. Explain. List the process details which ensure that anastrozole meets high purity specifications.
Anti‐Infective Medicines/Antiprotozoal Medicines/Antimalarial Medicines/For Curative Treatment
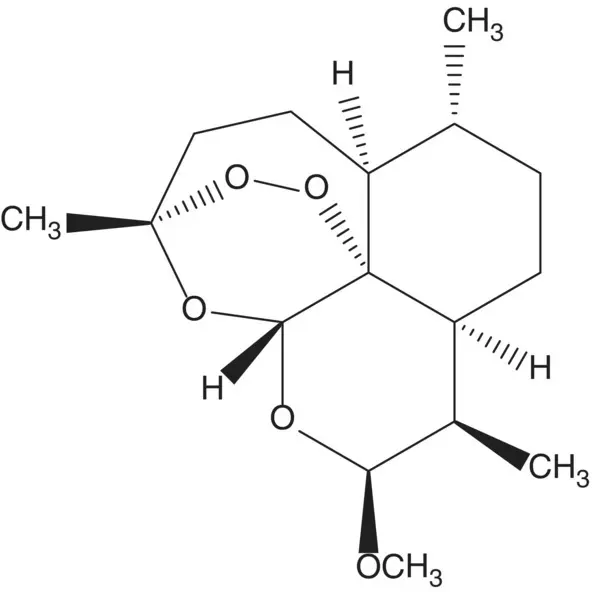
A single‐enantiomer molecule with multiple chiral carbons is often formed by modification of a natural product which has most or all of the chiral carbons already in place.
Discussion.Artemether (β‐artemether) is semisynthetic, it is manufactured in two steps from artemisinin. The methyl acetal of β‐artemether is formed by the acid‐catalyzed reaction of the hemiacetal (dihydroartemisinin) with methanol. The hemiacetal of dihydroartemisinin is formed by reduction of the ester of artemisinin. Artemisinin is a natural product isolated from the plant Artemisia annua or sweet wormwood.
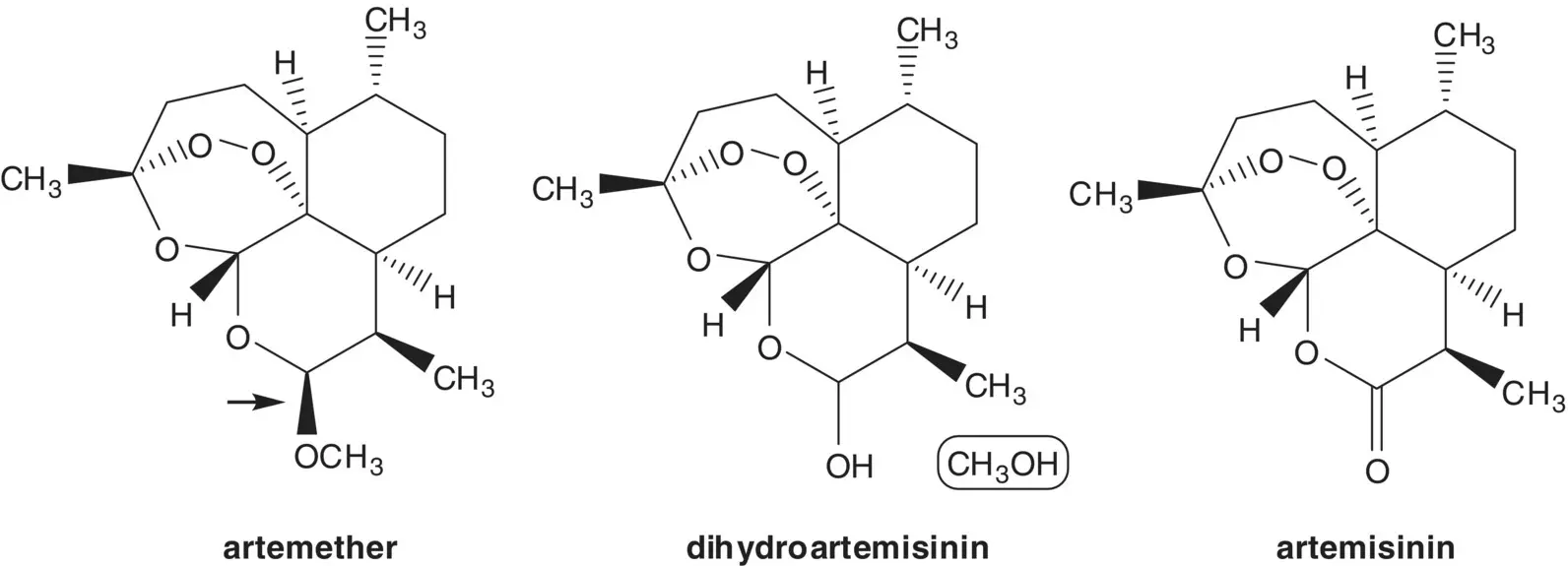
Artemisinin can also be manufactured in four steps from artemisinic acid. In the last step, a hydroperoxide is formed by α‐oxidation of an aldehyde with triplet oxygen. The aldehyde, hydroperoxide, ketone, and carboxylic acid then assemble to form artemisinin. The aldehyde and ketone are formed by cleavage of an allylic hydroperoxide ( Hock Rearrangement). The allylic hydroperoxide is formed from dihydroartemisinic acid ( Ene Reaction). Dihydroartemisinic acid is formed by reduction of artemisinic acid. Artemisinic acid is a natural product also isolated from the plant A. annua or sweet wormwood. Artemisinic acid is also produced by fermentation.
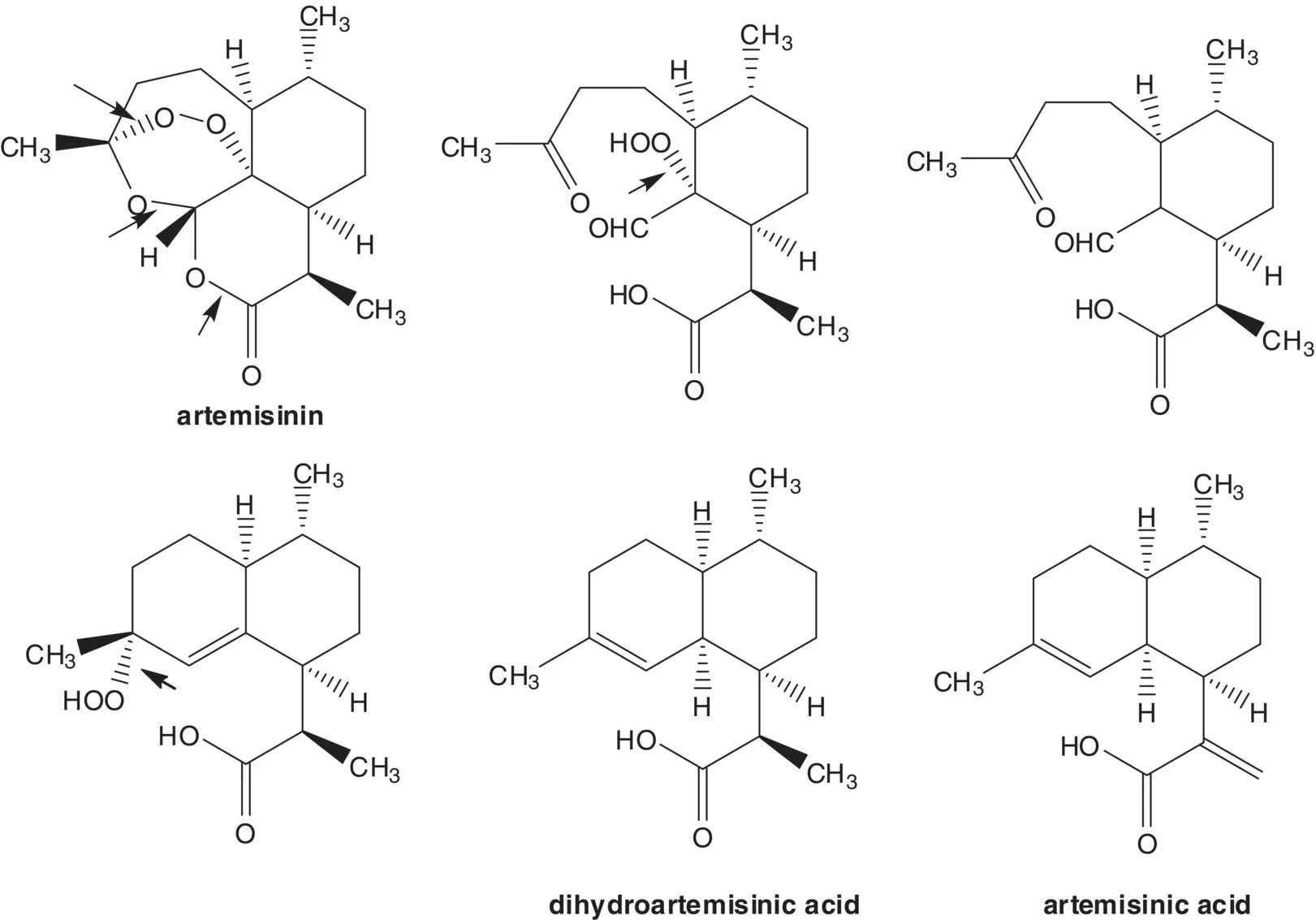
Draw the structures of four impurities which are likely to form in the conversion of dihydroartemisinin to artemether.
Anti‐Infective Medicines/Antiprotozoal Medicines/Antimalarial Medicines/For Curative Treatment
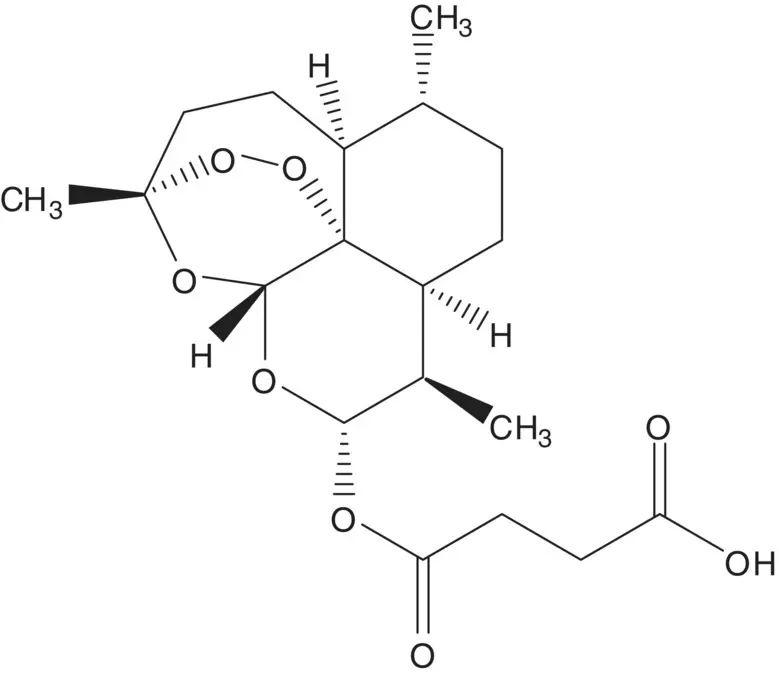
A single‐enantiomer molecule with multiple chiral carbons is often formed by modification of a natural product which has most or all of the chiral carbons already in place.
Discussion.Artesunate is semisynthetic, and it is manufactured in two steps from artemisinin. The ester is formed by reaction of the hemiacetal (dihydroartemisinin) with succinic anhydride. The hemiacetal of dihydroartemisinin is formed by reduction of the ester of artemisinin. Artemisinin is a natural product isolated from the plant A. annua or sweet wormwood.
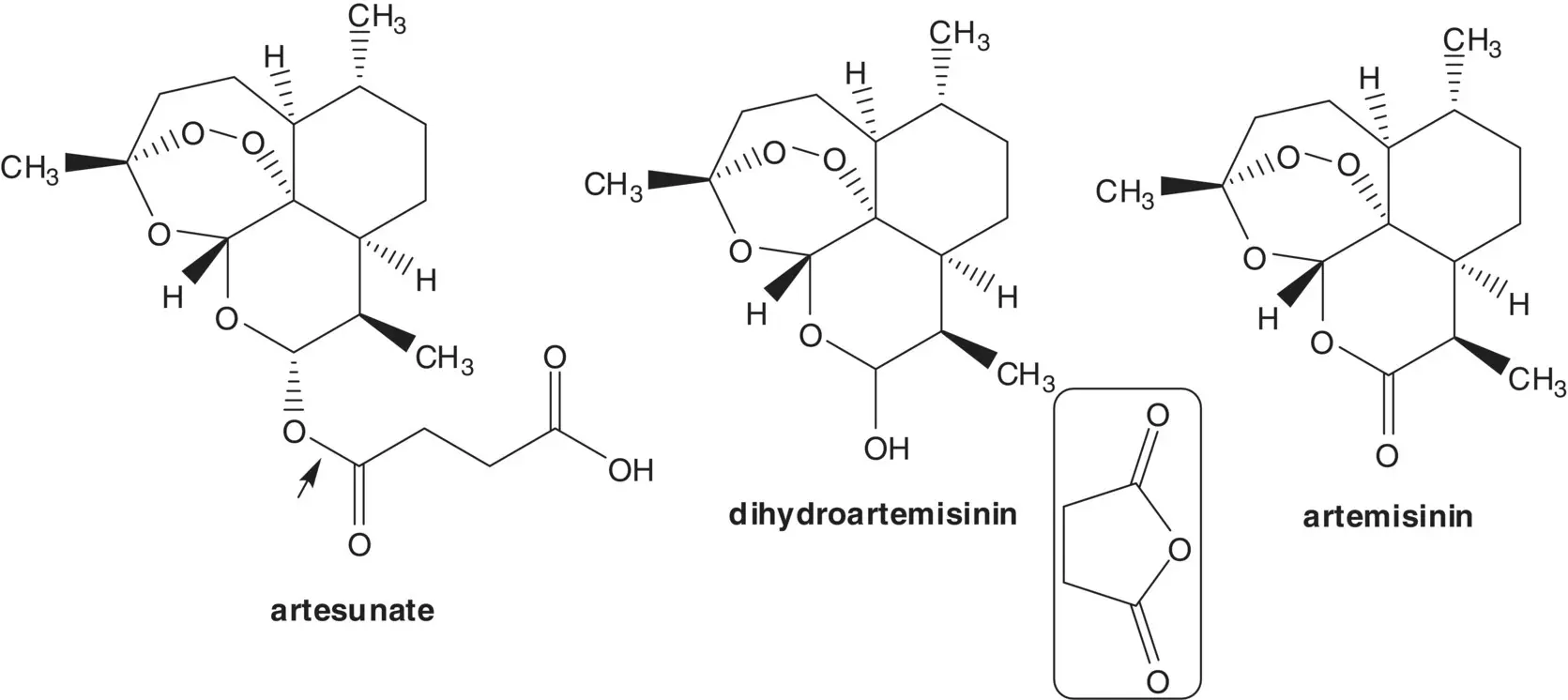
Artemisinin is also manufactured in four steps from artemisinic acid. In the last step, a hydroperoxide is formed by α‐oxidation of an aldehyde with triplet oxygen. The aldehyde, hydroperoxide, ketone, and carboxylic acid then assemble to form artemisinin. The aldehyde and ketone are formed by cleavage of an allylic hydroperoxide ( Hock Rearrangement). The allylic hydroperoxide is formed from the alkene ( Ene Reaction). The alkene, dihydroartemisinic acid, is formed by reduction of artemisinic acid. Artemisinic acid is a natural product also isolated from the plant A. annua or sweet wormwood. Artemisinic acid is also produced by fermentation.
Читать дальше
























