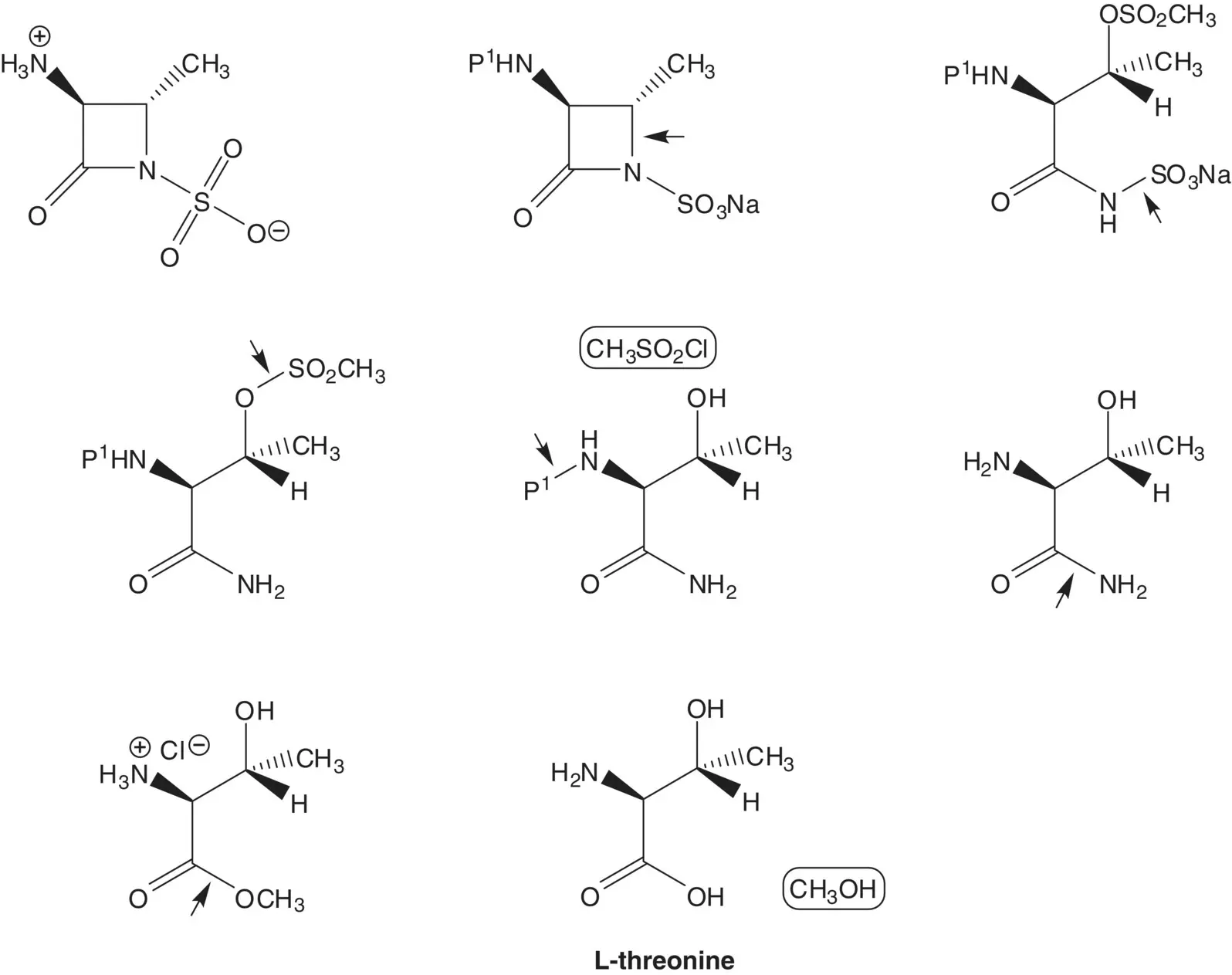
The thioester is formed from the carboxylic acid and 2,2′‐dithiobis(benzothiazole). The carboxylic acid is formed by hydrolysis of the ethyl ester. The O ‐alkyloxime is formed by O ‐alkylation of the oxime with tert ‐butyl α‐bromoisobutyrate. The thiazole ring is formed by reaction of an α‐bromoketone with thiourea ( Hantzsch Thiazole Synthesis). The α‐bromoketone is formed by bromination of the ketone. The oxime is formed by nitrosation of ethyl acetoacetate.
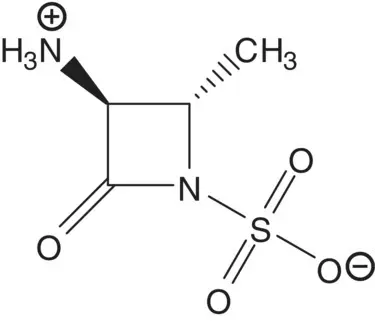
Draw the structures of the retrosynthetic analysis of an alternative route to the ammonium sulfamate zwitterion. Include the structures of the retrosynthetic analysis of any organic starting material(s) from petrochemical or biochemical raw materials. List the pros and cons for both routes and select one route to the ammonium sulfamate zwitterion as the preferred route.
B
Beclomethasone Dipropionate
Medicines Acting on the Respiratory Tract/Antiasthmatic and Medicines for Chronic Obstructive Pulmonary Disease
A single‐enantiomer molecule with multiple chiral carbons is often formed by modification of a natural product which has most or all of the chiral carbons already in place. A steroid with 16β‐methyl and 17α‐hydroxy substituents is often formed by ring‐opening of a 16α,17‐epoxide with a methylmagnesium halide.
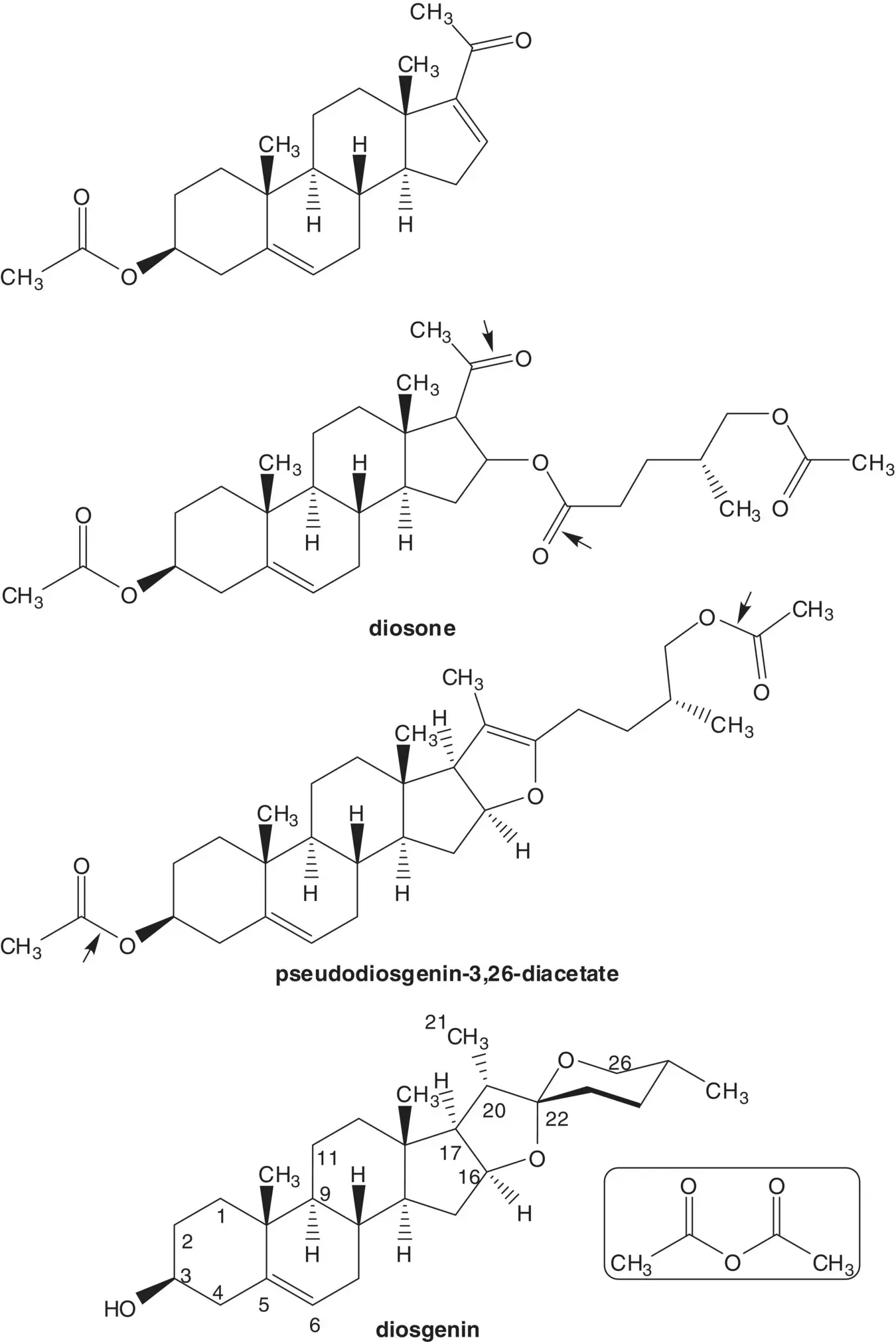
Discussion.Beclomethasone dipropionate is manufactured in 3 steps from beclomethasone, 12 steps from 16β‐methylpregnane‐3β,17α,21‐triol 21‐acetate, 19 steps from 16‐dehydropregnenolone acetate, and 22 steps from diosgenin. Diosgenin is a phytosteroid sapogenin isolated from the tubers of Dioscorea wild yam.
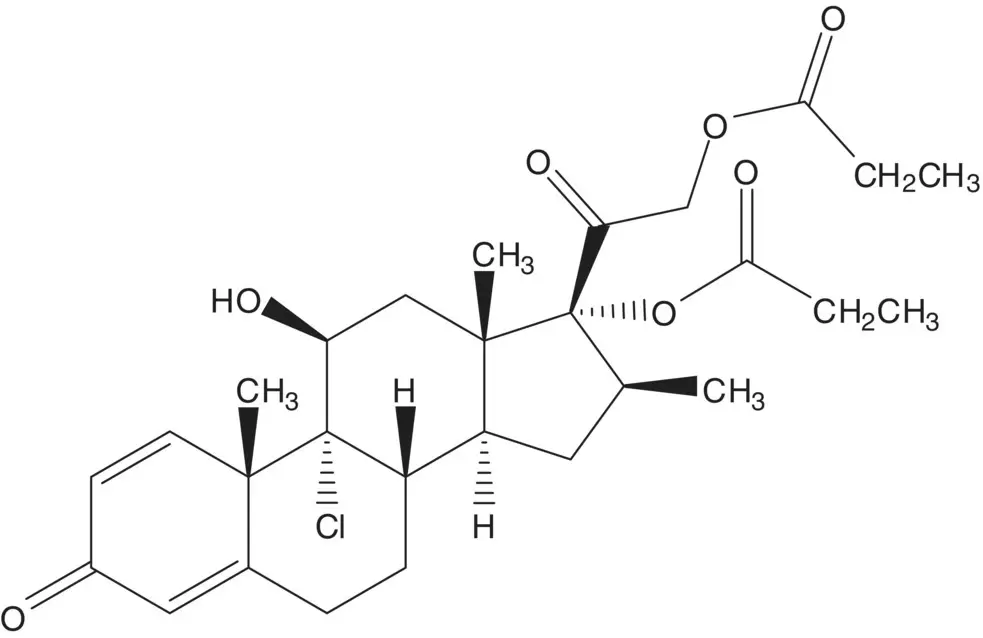
Beclomethasone 17α,21‐dipropionate is formed from the 17α‐propionate and propanoic anhydride. The 17α‐propionate is formed via the 17α,21‐orthoester. The 17α,21‐orthoester is formed by reaction of beclomethasone and trimethyl orthopropionate (1,1,1‐trimethoxypropane).
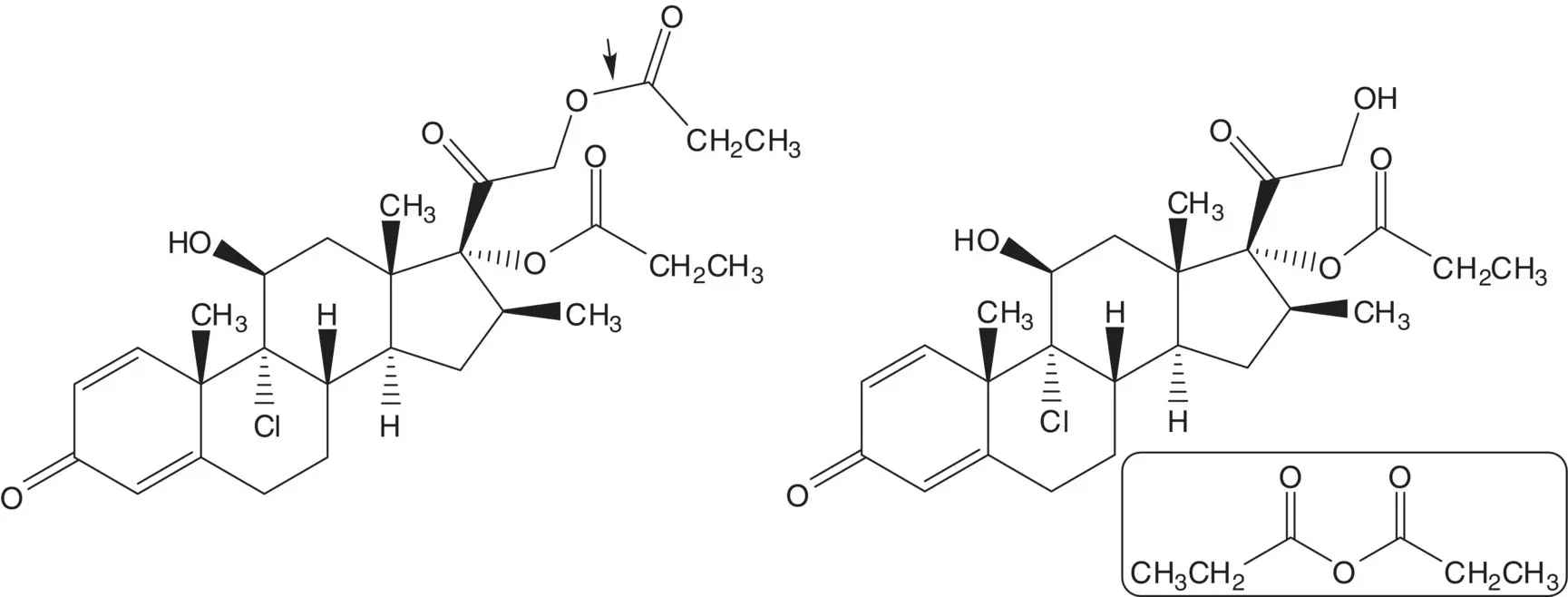
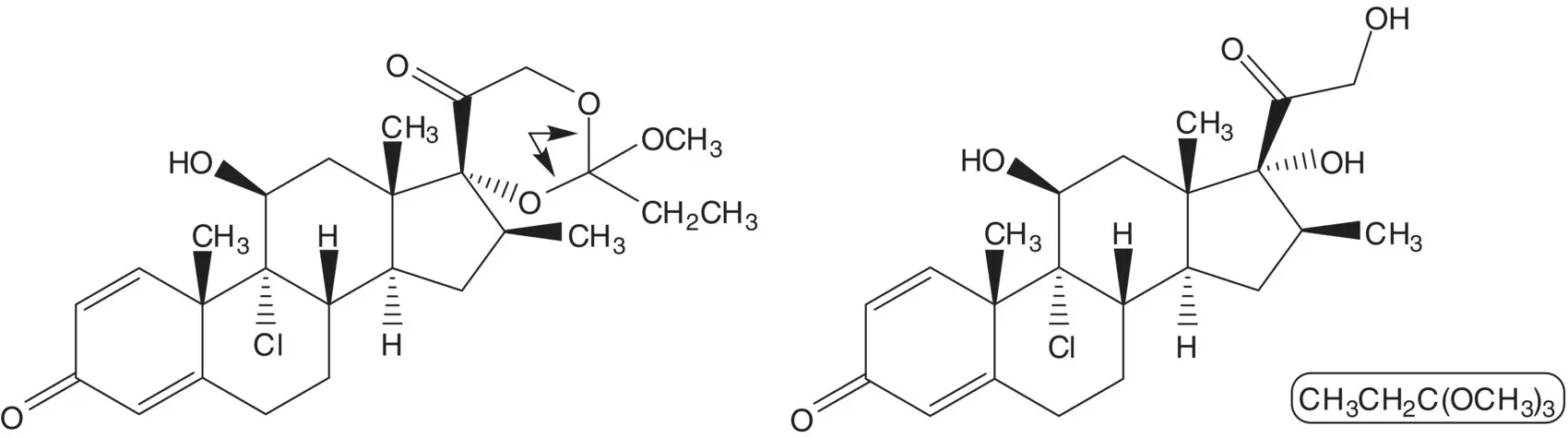
Chlorine is introduced at C9 on the α–face by ring‐opening of the 9β,11‐epoxide with hydrogen chloride. The 9β,11‐epoxide is formed from the bromohydrin. The 11β‐alcohol of the bromohydrin is formed in situ by hydrolysis of the formate ester. The 21‐alcohol is also released by hydrolysis of the carbonate ester. The bromohydrin formate is formed from the 9(11)‐alkene. The 9(11)‐alkene is formed by dehydration of the 11α‐alcohol. The 21‐alcohol is protected as a carbonate.
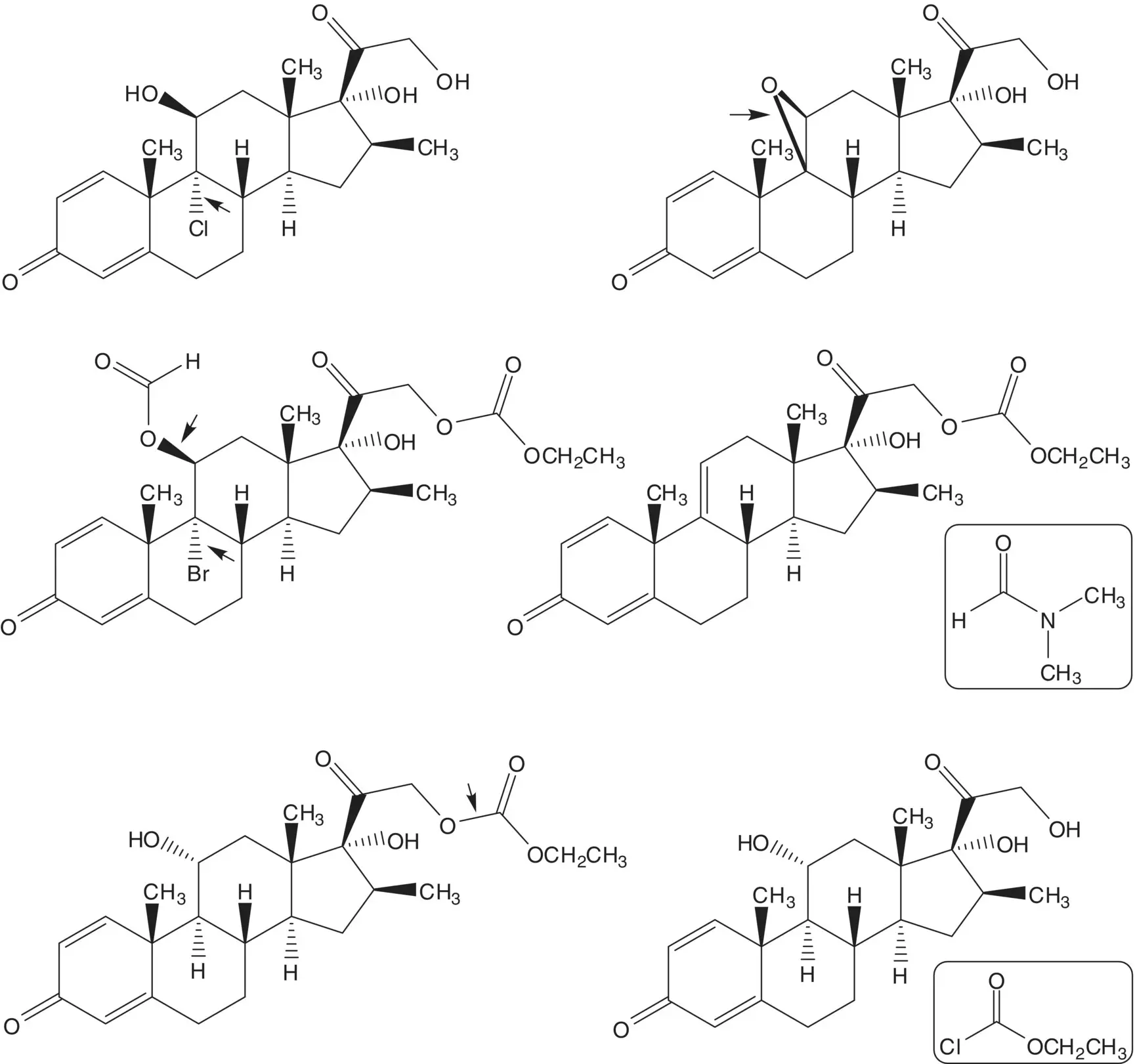
The 11α‐alcohol is formed by microbial oxidation. The C21 acetate ester is hydrolyzed during the microbial oxidation. The 1,4‐diene‐3‐one is formed from the 2α,4α‐dibromo‐3‐one by double elimination. The 2α,4α‐dibromo‐3‐one is formed by α‐bromination of the C3 ketone. The C3 ketone is formed by oxidation of the 3β‐alcohol of 16β‐methylpregnane‐3β,17α,21‐triol 21‐acetate.
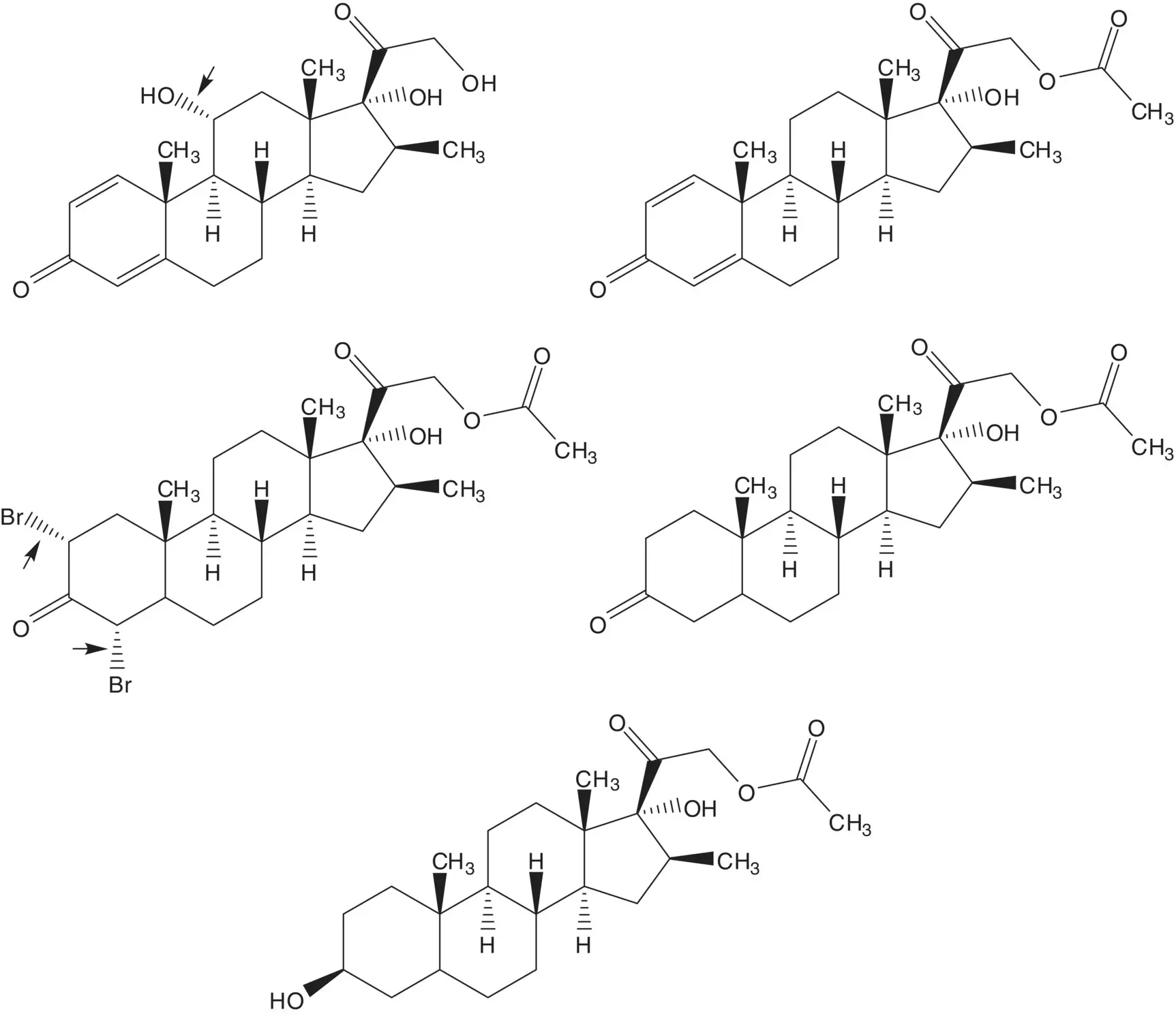
The acetate ester is formed by displacement of bromide by acetate. The α‐bromoketone is formed by α‐bromination of the C20 ketone. The C20 ketone is released by acetal hydrolysis. The 16β‐methyl substituent is introduced by ring‐opening of the 16α,17‐epoxide with methylmagnesium bromide ( Grignard Reaction). Methylmagnesium bromide is formed from bromomethane. The 5,6‐alkene is reduced by catalytic hydrogenation. (What is the stereochemistry of the new chiral carbon at C5? Add this stereochemical feature to the structures in the retrosynthetic analysis.) The C20 ketone is protected as an acetal by reaction with ethylene glycol. The 16α,17‐epoxide is formed by epoxidation of the 16‐alkene of 16‐dehydropregnenolone acetate. The acetate ester is hydrolyzed under the epoxidation conditions.
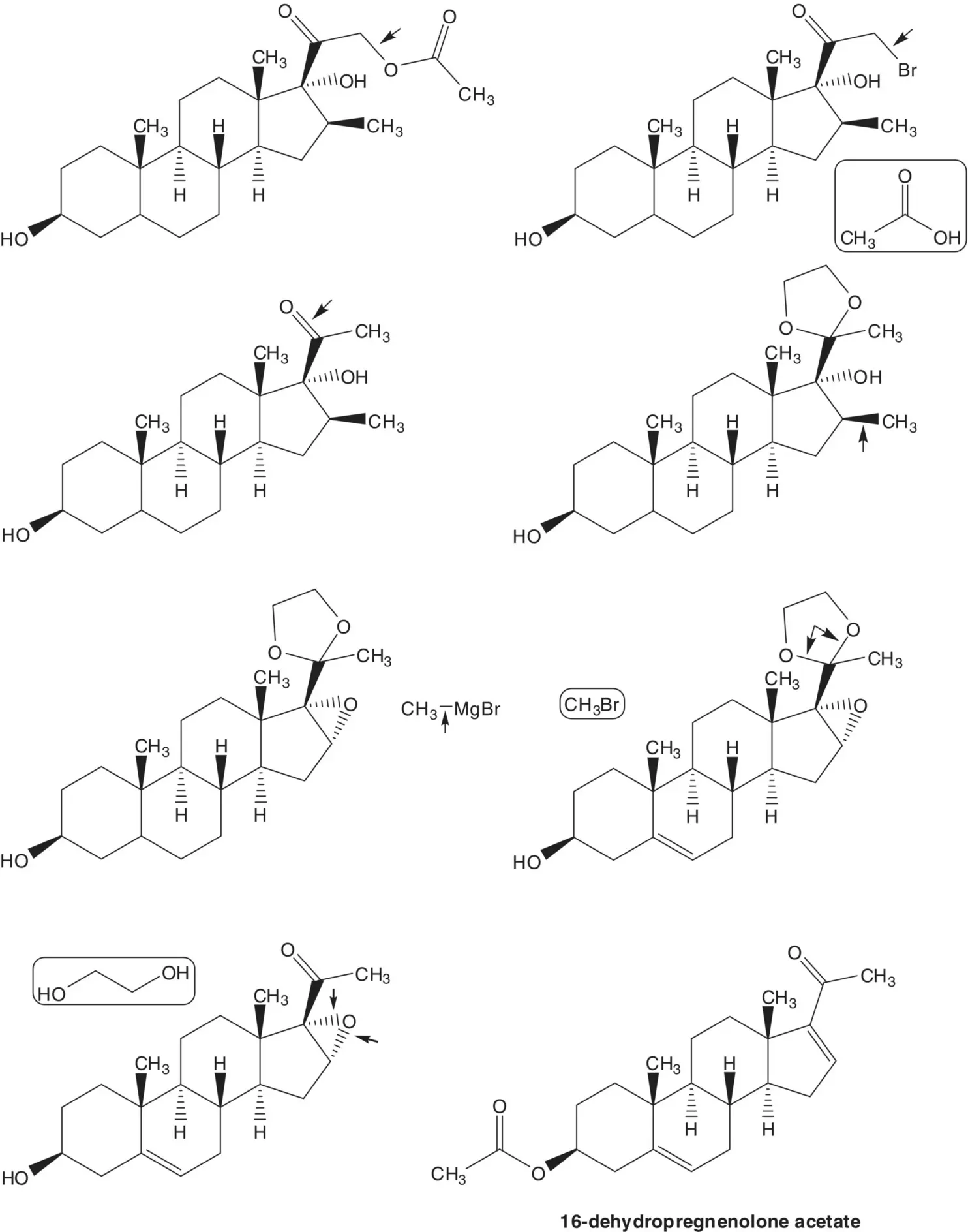
The 16‐alkene of 16‐dehydropregnenolone acetate is formed from diosone by β‐elimination. Diosone is formed by oxidation of the 20(22)‐alkene of pseudodiosgenin‐3,26‐diacetate. Pseudodiosgenin 3,26‐diacetate is formed by reaction of diosgenin with acetic anhydride. The three‐step synthesis of 16‐dehydropregnenolone acetate from diosgenin by acetylation, oxidation, and elimination is known as the Marker Degradation.
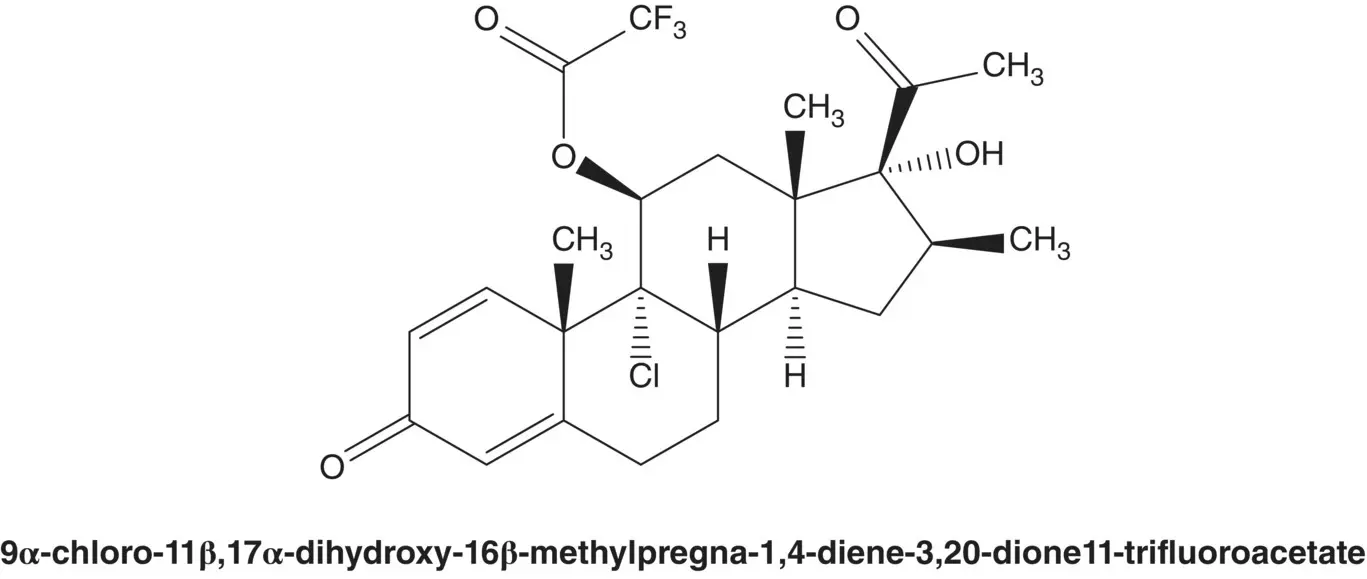
Draw the structures of the retrosynthetic analysis of one alternative route to beclomethasone dipropionate via the 11β‐trifluoroacetate intermediate.
Anti‐infective Medicines/Antibacterials/Antituberculosis Medicines
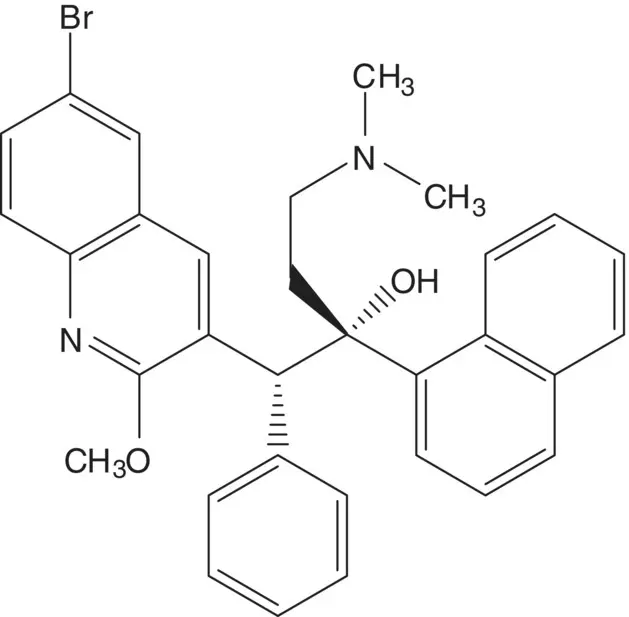
A tertiary alcohol is often formed by addition of an organometallic reagent RLi or RMX (M = Mg, Zn, X = Cl, Br, I) to a ketone.
Discussion.Bedaquiline, the (1 R ,2 S )‐enantiomer, is separated from a 1 : 1 mixture of (1 R ,2 S )‐ and (1 S ,2 R )‐enantiomers by resolution in the final step. (Draw the structures of the chiral acids used for the resolution. What is the yield of bedaquiline for the two‐step resolution process associated with each chiral acid?) The addition of an alkyllithium to a ketone results in a mixture of four stereoisomers. The undesired (1 S ,2 S )‐ and (1 R ,2 R )‐enantiomers are separated from the mixture by crystallization. The desired (1 R ,2 S )‐ and (1 S ,2 R )‐enantiomers are then isolated by crystallization. (What is the highest diastereoselectivity for the addition reaction? What reagents and reaction conditions are associated with the highest diastereoselectivity?) The alkyllithium is formed from 3‐benzyl‐6‐bromo‐2‐methoxyquinoline. The β–dimethylamino ketone is formed from 1‐acetonaphthone, formaldehyde, and dimethylamine ( Mannich Reaction).
Читать дальше

























