Discussion.Atropine, a 1 : 1 mixture of the tropane alkaloids ( R )‐hyoscyamine and ( S )‐hyoscyamine, is usually produced by extraction from the plants Atropa belladonna , Datura stramonium , or Duboisis myoporoides .
Atropine can also be synthesized from tropic acid and tropinone. In the final step of the synthesis, the primary alcohol is released by acetate ester hydrolysis. The tropic acid ester is formed from the acid chloride and the alcohol, 3‐tropanol (tropine).
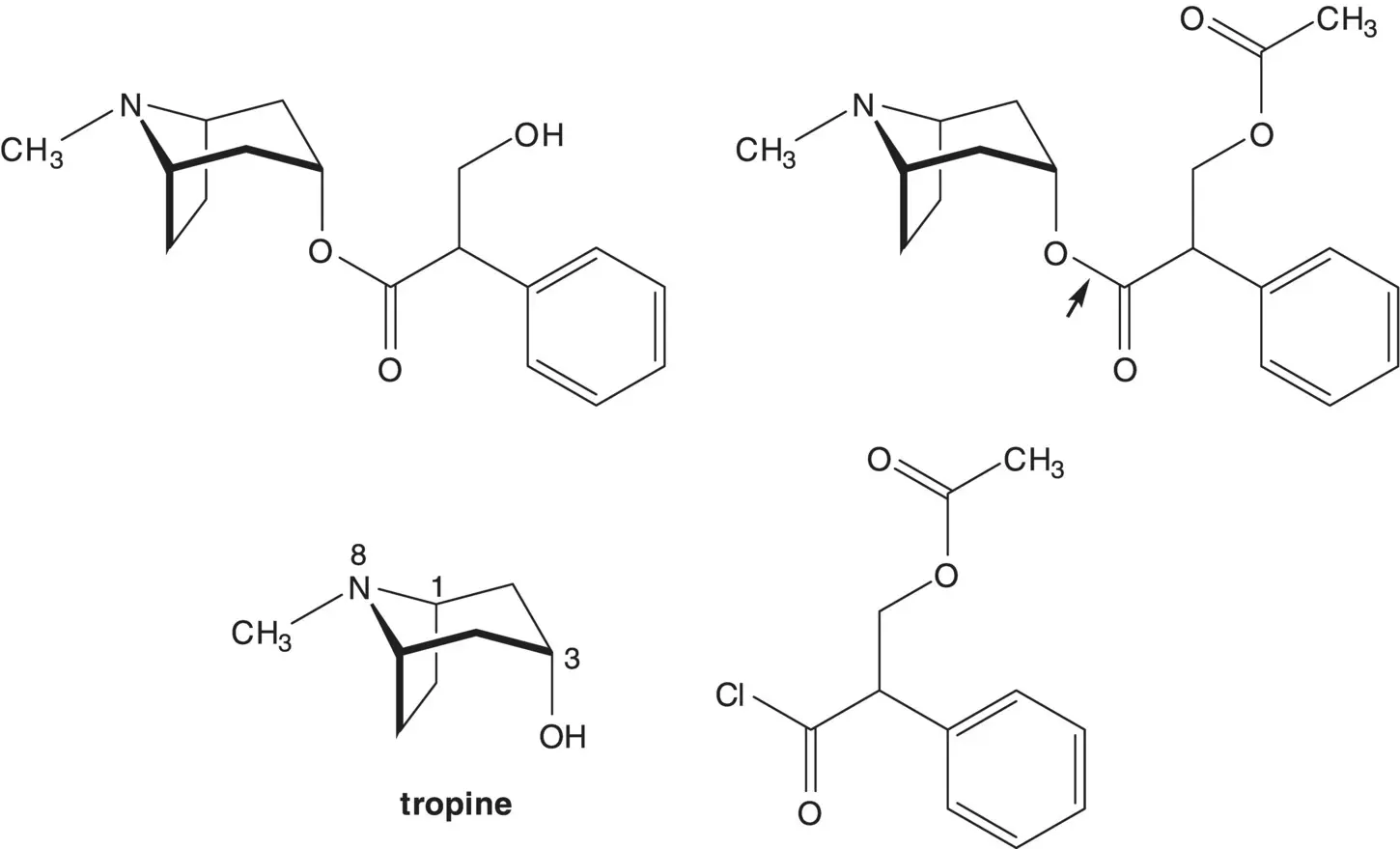
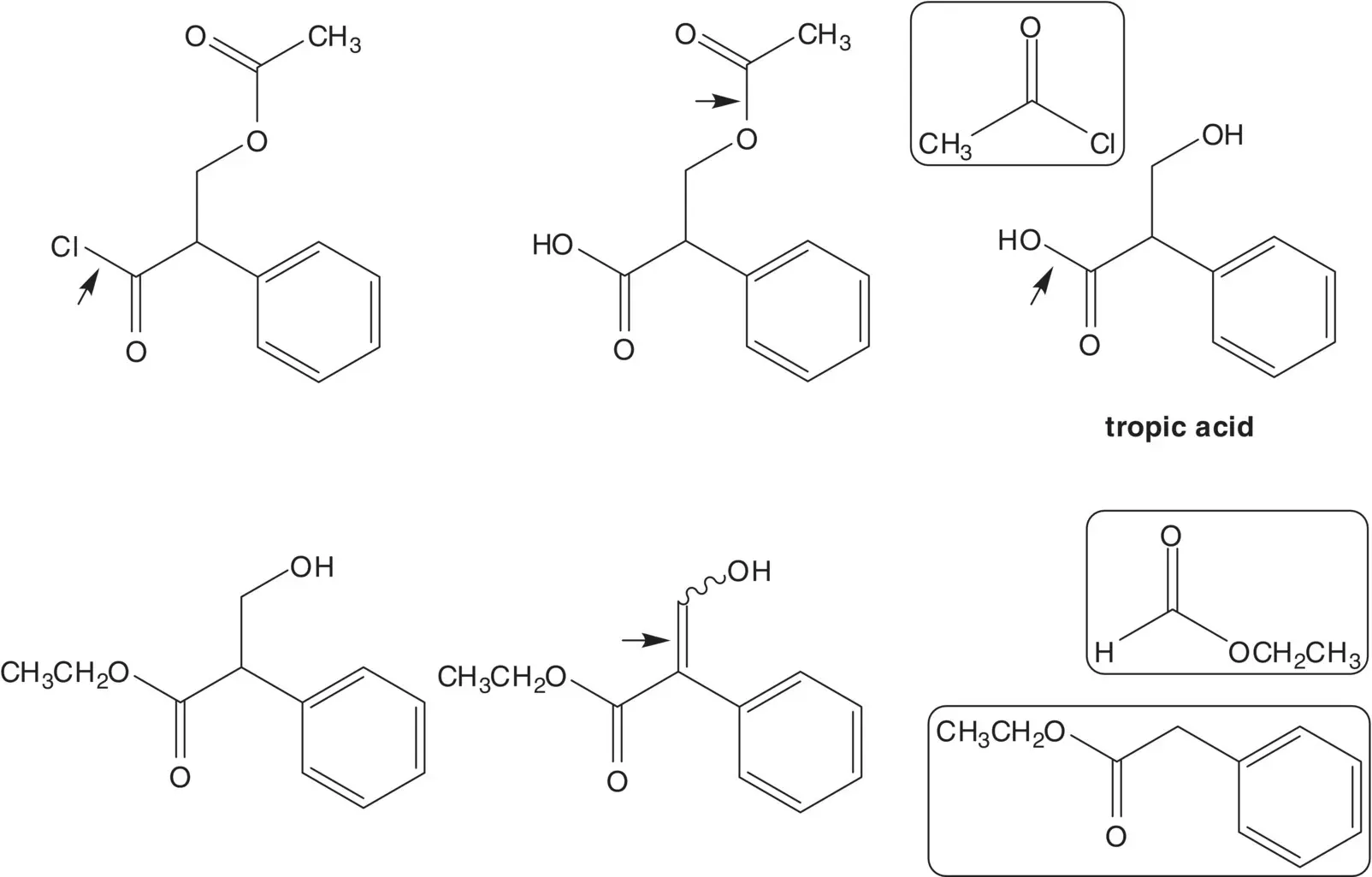
In a one‐pot procedure, the acetate ester is formed from acetyl chloride and the primary alcohol of tropic acid then the acid chloride is then formed from the carboxylic acid. Tropic acid is formed by hydrolysis of the ethyl ester. The α‐hydroxymethyl ester is formed by reduction of the α‐formyl ester. The α‐formyl ester is formed from ethyl phenylacetate and ethyl formate (mixed Claisen Condensation).
The axial alcohol of tropine is formed by reduction of the ketone. Tropinone is efficiently assembled in a single step from methylamine, 2,5‐dimethoxytetrahydrofuran, and 1,3‐acetonedicarboxylic acid ( Robinson–Schopf Reaction). 1,3‐Acetonedicarboxlic acid is formed by oxidative decarboxylation of citric acid. Citric acid is produced by fermentation.
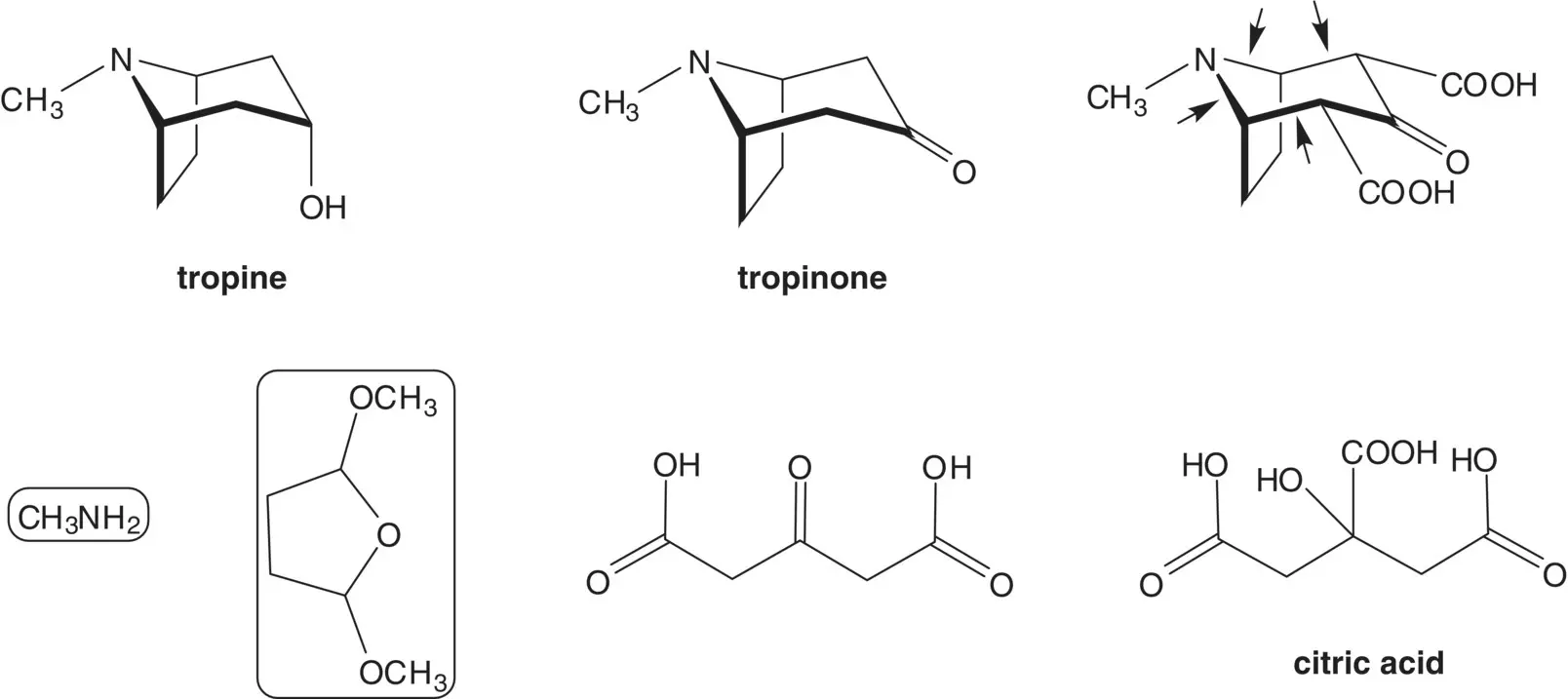
The yield in the first one‐pot synthesis of tropinone, described by Robinson in 1917, was just 17%. After a century of process development, the yield for the one‐pot synthesis is now 90%! List the references for the available procedures, the process modification(s) made, and the tropinone yield. Which process modification had the greatest impact on the yield?
Antineoplastics and Immunosuppressives/Immunosuppressive Medicines
Medicines for Diseases of Joints/Disease‐Modifying Agents Used in Rheumatoid Disorders
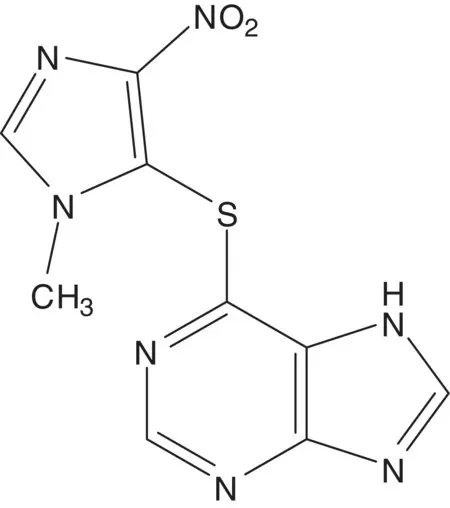
An aromatic thioether is often formed by displacement of chloride or bromide by a thiol.
Discussion.The thioether is formed in the final step by displacement of chloride from 5‐chloro‐1‐methyl‐4‐nitroimidazole by 6‐mercaptopurine. 5‐Chloro‐1‐methyl‐4‐nitroimidazole is formed by nitration of 5‐chloro‐1‐methylimidazole. 5‐Chloro‐1‐methylimidazole is formed from N,N ′‐dimethyloxamide ( Wallach Imidazole Synthesis). The oxamide is formed from diethyl oxalate and methylamine. 6‐Mercaptopurine is formed from hypoxanthine.
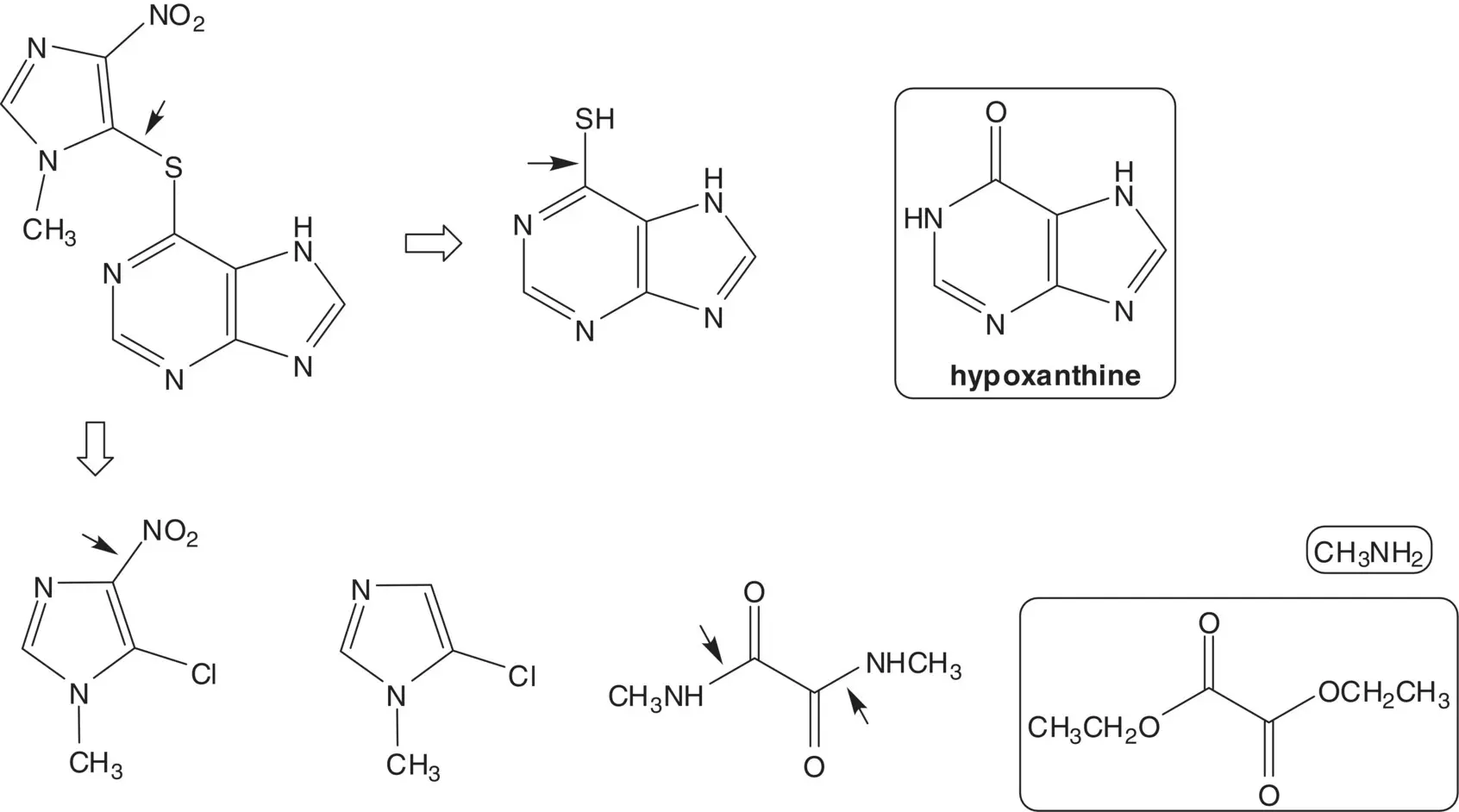
Draw the structures of a retrosynthetic analysis of one alternative route to the thioether by nucleophilic aromatic substitution of chloride from 6‐chloropurine. Compare the two routes and select one route as the preferred route.
Anti‐Infective Medicines/Antibacterials/Other Antibacterials
Ophthalmological Medicines/Anti‐Infective Agents
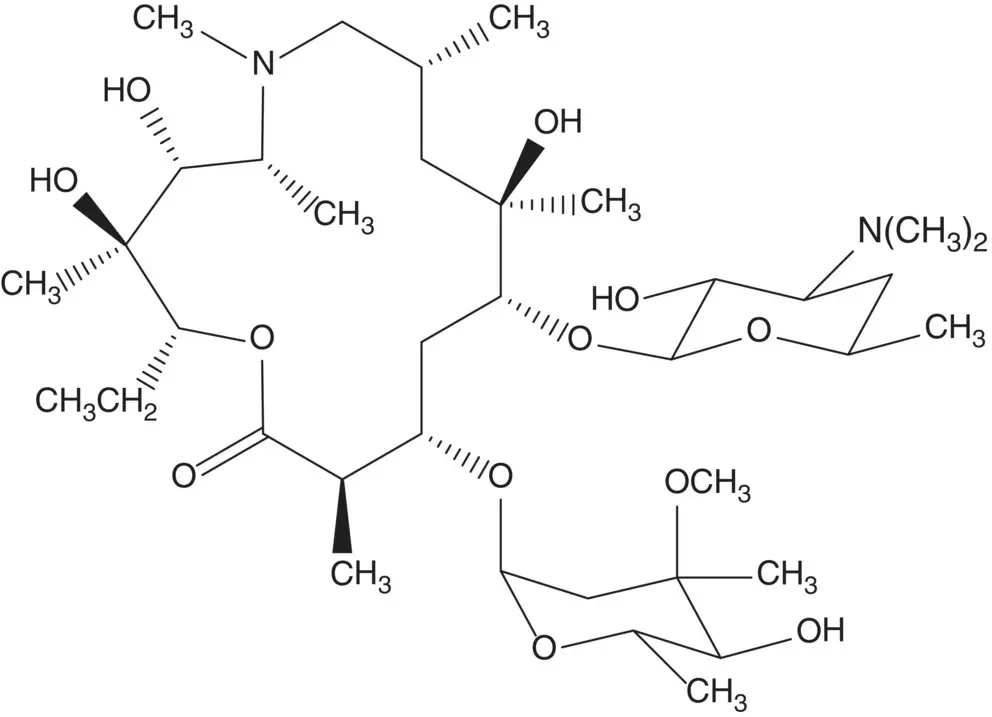
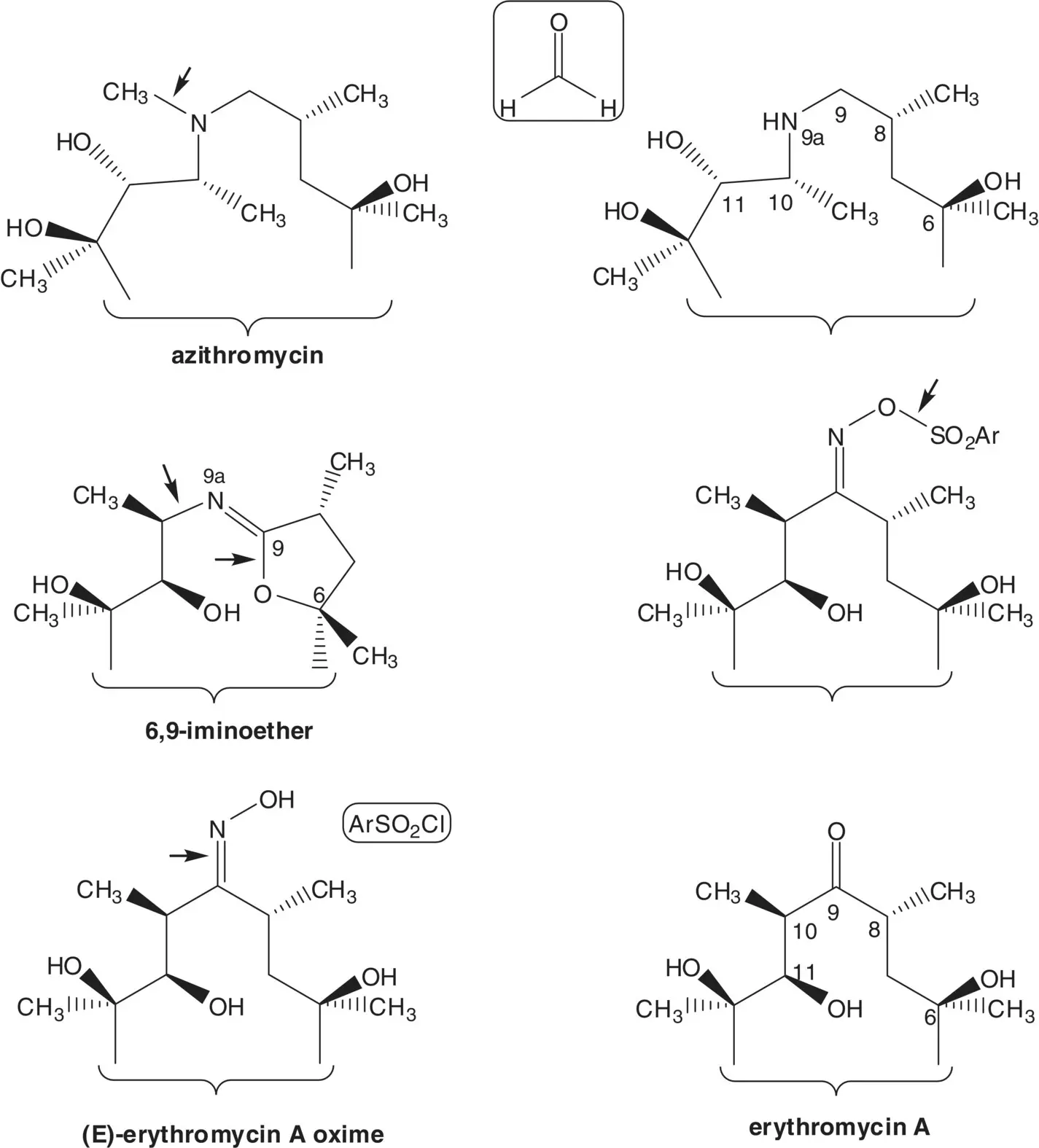
Macrolide antibiotics are produced by fermentation or are semisynthetic. The process for manufacture of a semisynthetic macrolide antibiotic often begins with conversion of the C9 ketone of erythromycin A to the oxime.
Discussion.Azithromycin is semisynthetic. Azithromycin is formed from the essential medicine erythromycin A which is produced by fermentation.

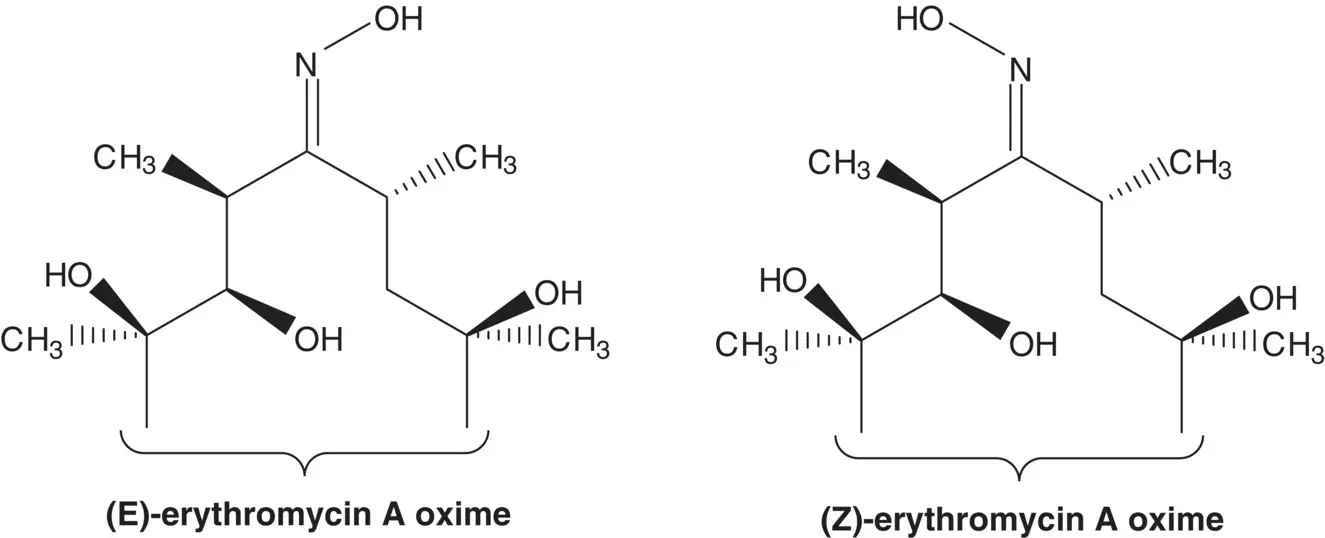
The tertiary amine of azithromycin is formed in the final step by methylation of the secondary amine with formaldehyde and formic acid ( Eschweiler–Clarke Reaction). The secondary amine (9‐deoxo‐9a‐aza‐homoerythromycin) is formed by reduction of an iminoether. The 6,9‐iminoether (the iminoether involving the hydroxyl group at C6 and carbon at C9) is formed by rearrangement of an ( E )‐ O ‐arylsulfonyl oxime ( Beckmann Rearrangement). The ( E )‐ O ‐arylsulfonyloxime is formed from the ( E )‐oxime and an arylsulfonyl chloride (List the arylsulfonyl chlorides and ( O )‐arylsulfonyloxime yields). ( E )‐Erythromycin A oxime is formed from erythromycin A. (How is ( E )‐erythromycin A oxime separated from ( Z )‐erythromycin A oxime?)
( Z )‐Erythromycin A oxime can be prepared from ( E )‐erythromycin A oxime. Draw structures for the products formed as ( Z )‐erythromycin A oxime is carried through the same sequence used to convert ( E )‐erythromycin A oxime to azithromycin.
Anti‐Infective Medicines/Antibacterials/β‐Lactam Medicines
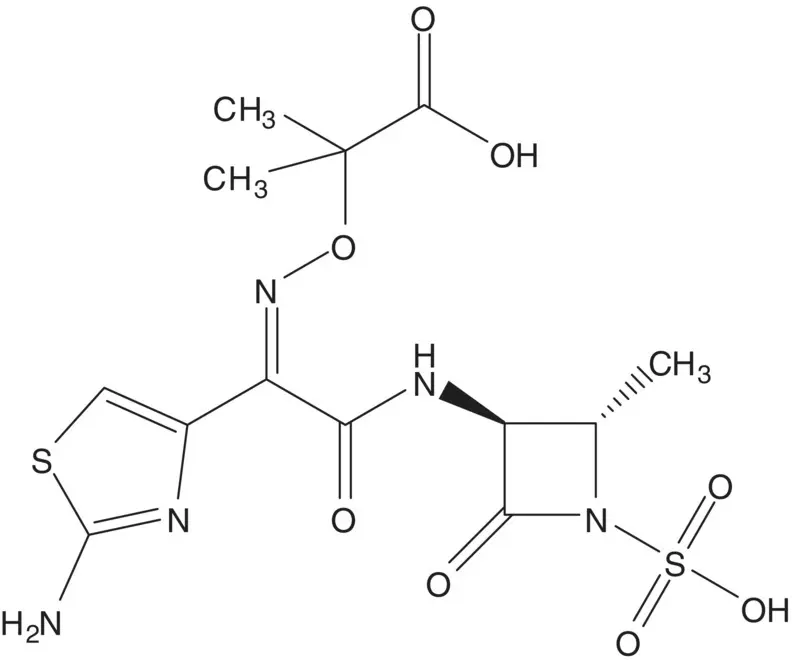
A single‐enantiomer molecule with multiple chiral carbons is often formed by modification of a natural product which has most or all of the chiral carbons already in place.
Discussion.In the final step, the carboxylic acid of aztreonam is released by hydrolysis of the tert ‐butyl ester. The amide bond near the center of the molecule is formed by reaction of the amine of the ammonium sulfamate zwitterion with a thioester.
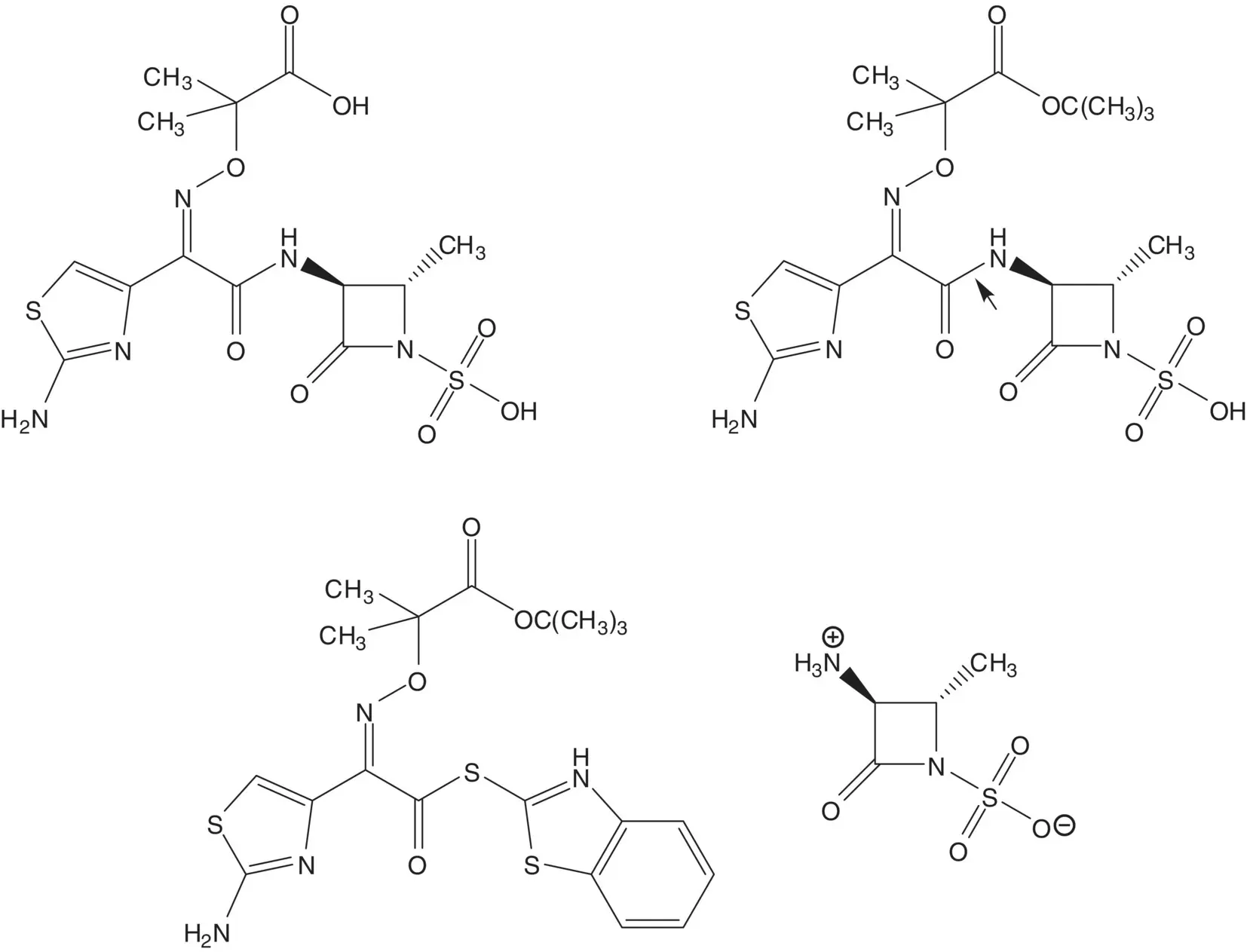
The amine of the ammonium sulfamate zwitterion is released by cleavage of the protecting group. The β‐lactam ring is formed by displacement of the secondary methanesulfonate by the N ‐acylsulfamate nitrogen. The N ‐acyl sulfamate is formed from the amide. The methanesulfonate is formed from the alcohol. The amine of L‐threonine amide is protected (List the protecting groups. Select one protecting group to use in the analysis). L‐Threonine amide is formed from L‐threonine methyl ester. L‐Threonine methyl ester hydrochloride is formed from L‐threonine ( Fischer Esterification). L‐Threonine is produced by fermentation.
Читать дальше























