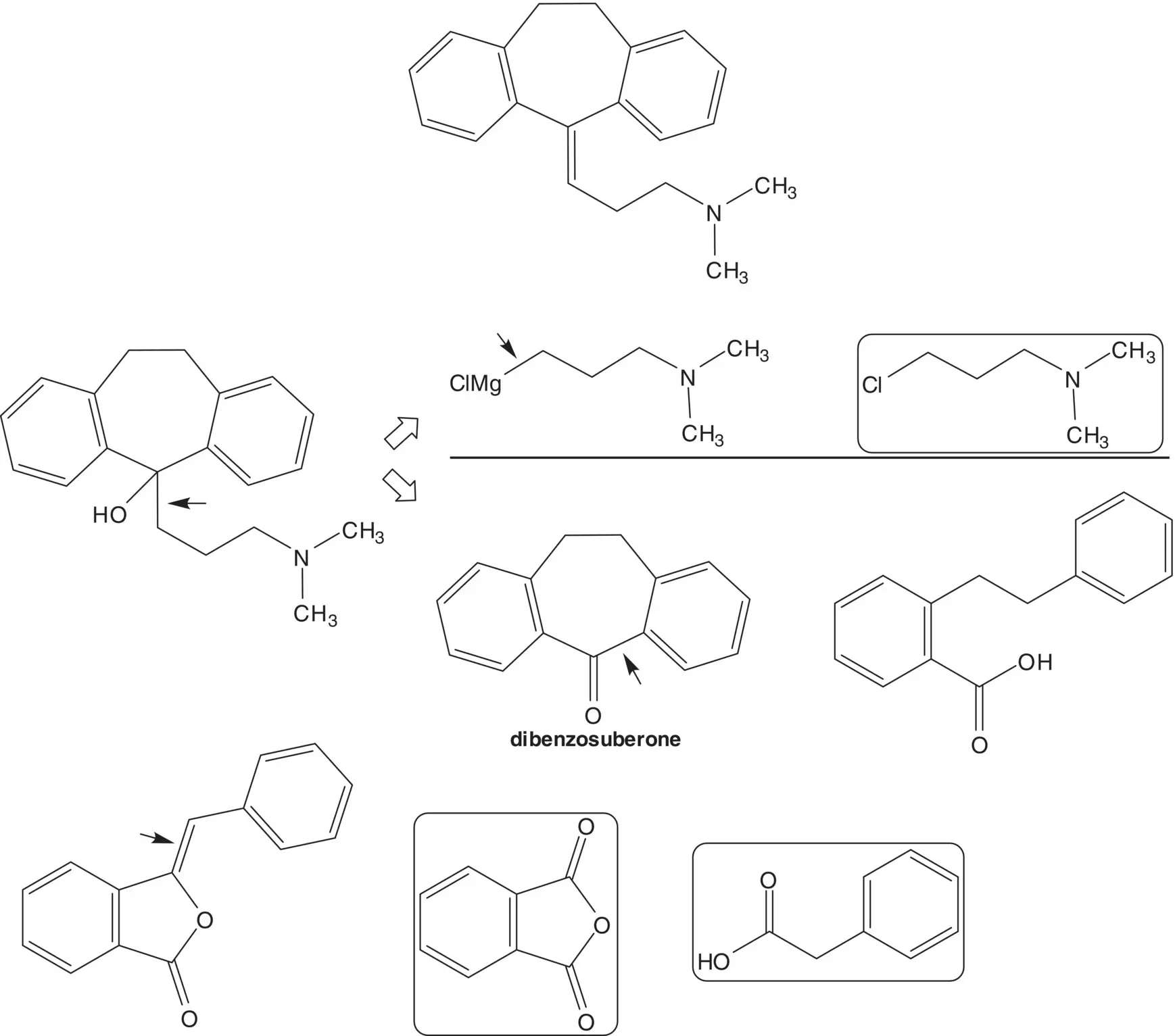
Dibenzosuberone is also formed from 2‐bromobenzyl bromide and carbon dioxide ( Parham Cyclization). List the pros and cons for the two dibenzosuberone routes and select one route as the preferred route.
Cardiovascular Medicines/Antihypertensive Medicines
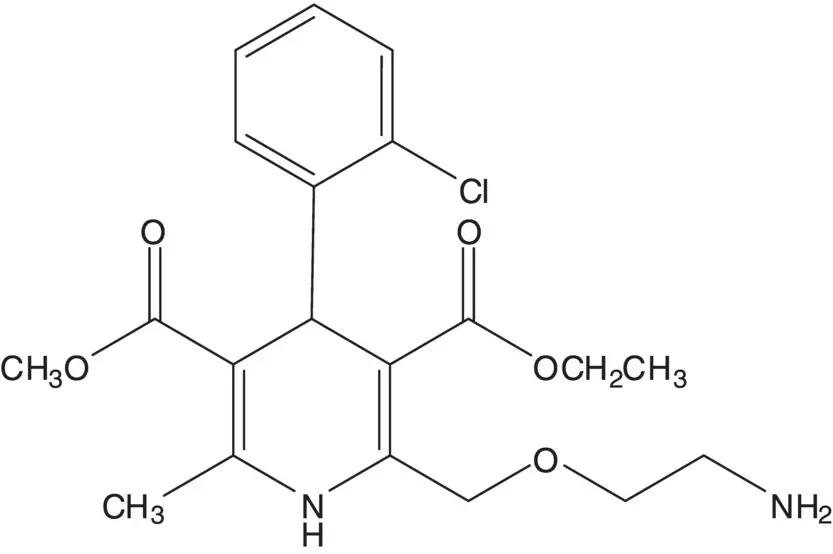
A dihydropyridine is often formed by Hantzsch Synthesis. The key step in the Hantzsch Synthesis is formation of a C3─C4 bond of the ring by Michael Addition of an enamine to an α,β‐unsaturated ketone or ester.
Discussion.While the ( S )‐enantiomer is a thousand times more active than the ( R )‐enantiomer, amlodipine is sold as the racemate. Racemic amlodipine is constructed in just four steps! The primary amine is formed using a Gabriel Synthesis. The final step is release of the primary amine from the phthalimide. The 1,4‐dihydropyridine is formed from methyl 3‐aminocrotonate and an enone by C─C bond formation ( Michael Addition) followed by C─N bond formation to close the ring ( Hantzsch Dihydropyridine Synthesis). The enone is formed by condensation of a β‐ketoester with 2‐chlorobenzaldehyde ( Knoevenagel Condensation). The ether on C4 of the β‐ketoester is formed by chloride displacement from ethyl 4‐chloro‐3‐oxobutanoate by the alcohol of N ‐(2‐hydroxyethyl)phthalimide ( Williamson Ether Synthesis).
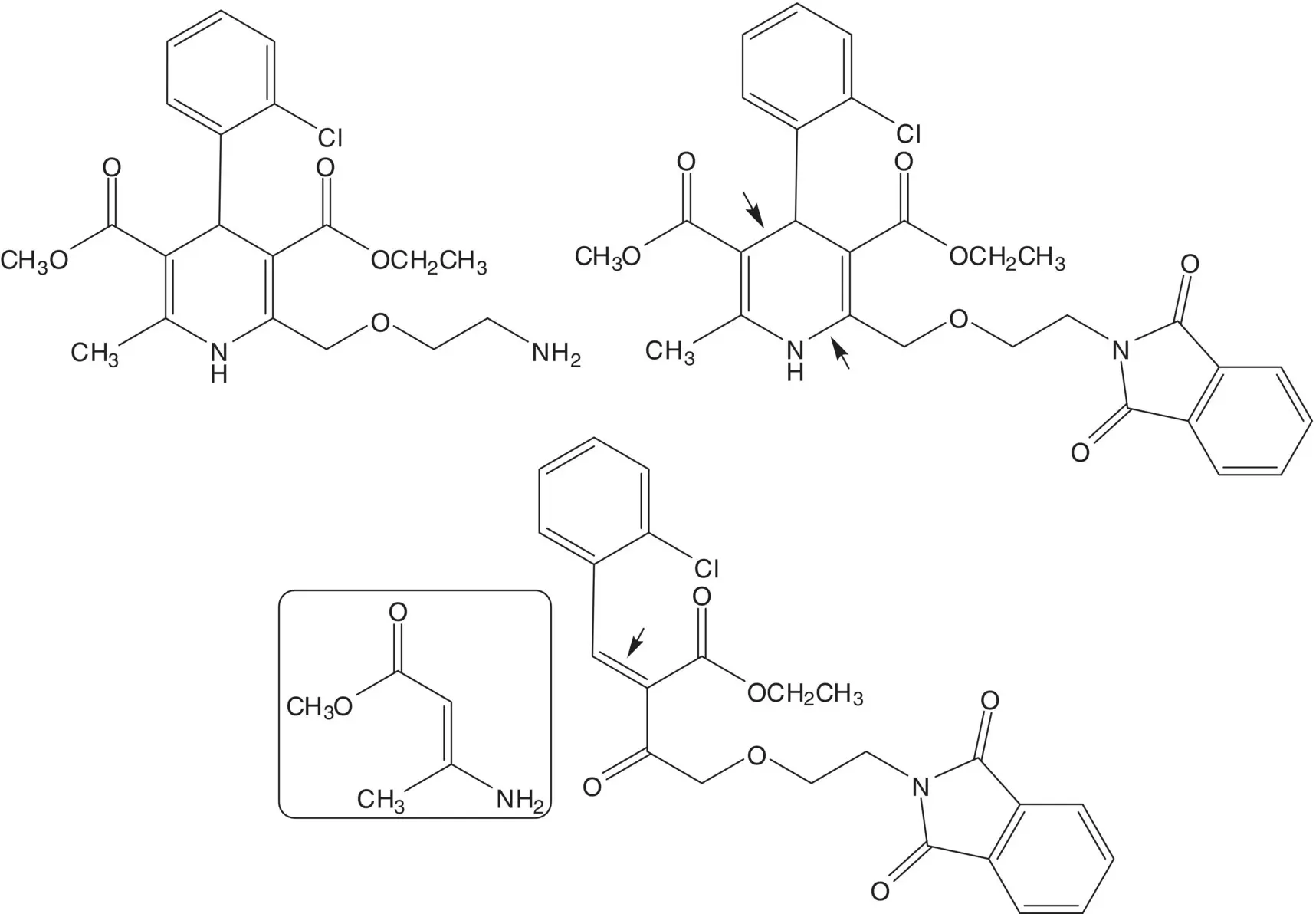
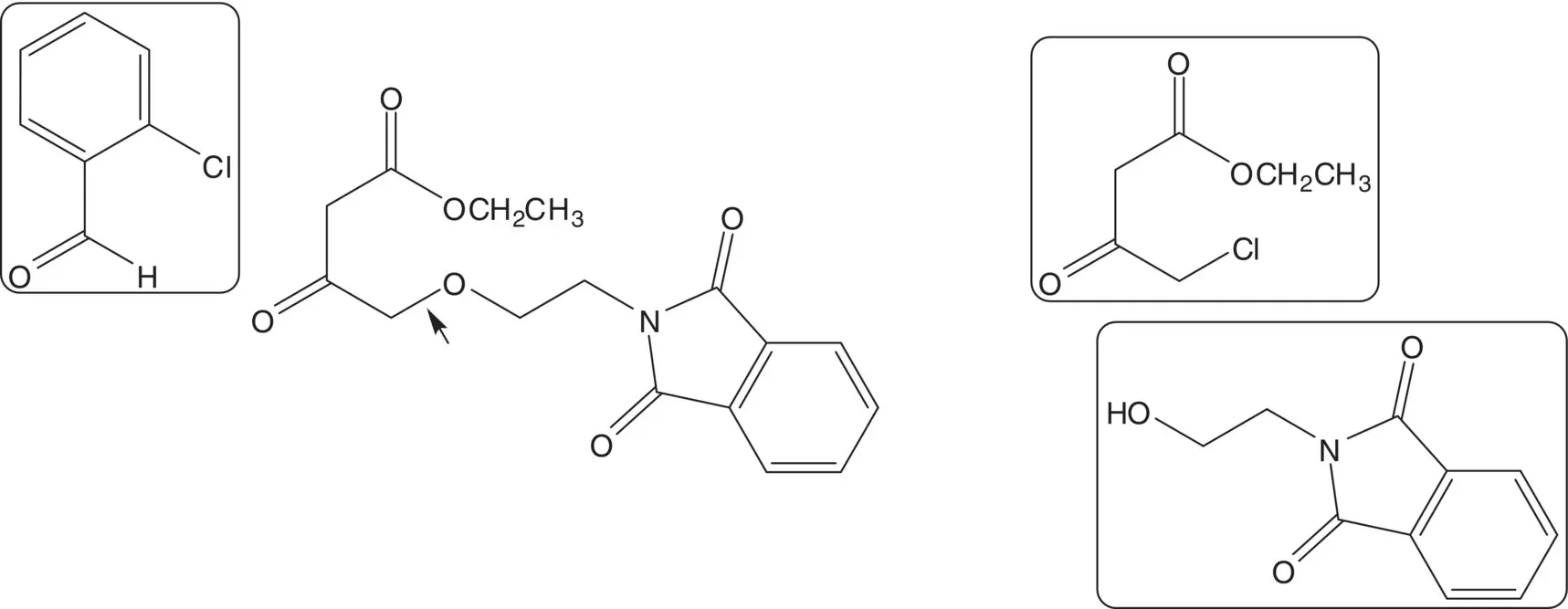
List the amine protecting groups that have been used in alternative syntheses of amlodipine. List the conditions used and amlodipine yields obtained in deprotection of each group in the final step. Why is the phthalimide group preferred?
Anti‐Infective Medicines/Antiprotozoal Medicines/Antimalarial Medicines/For Curative Treatment
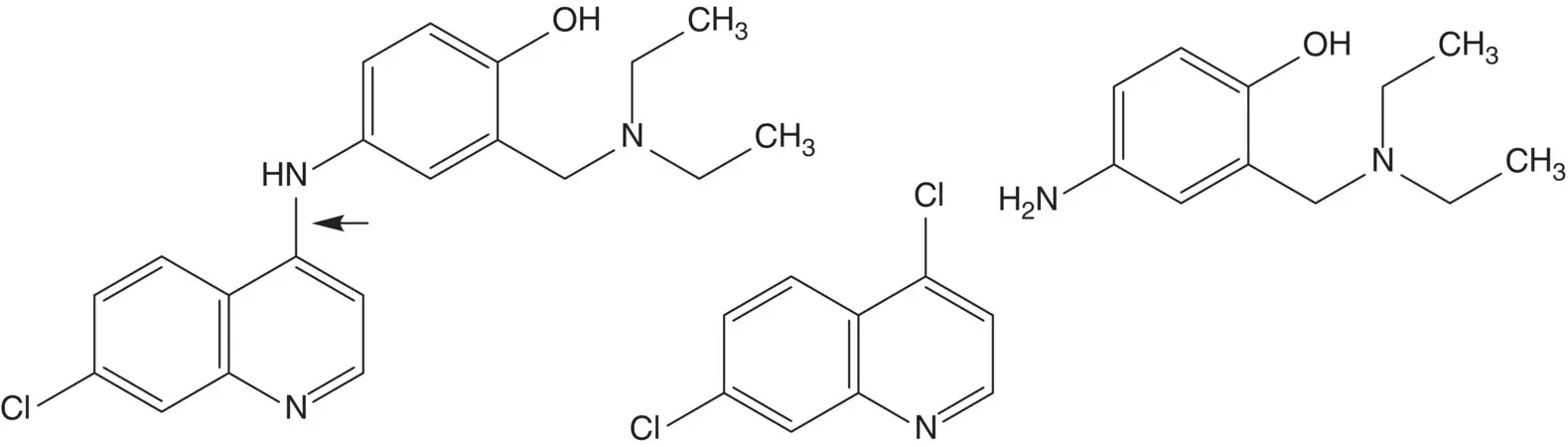
A nitrogen substituent at C2 or C4 on a quinoline ring is often introduced by displacement of chloride. The substitution is facilitated by the quinoline ring nitrogen and can be further facilitated by an electron‐withdrawing group (NO 2 , SO 2 R, COOR, CN) on C3.
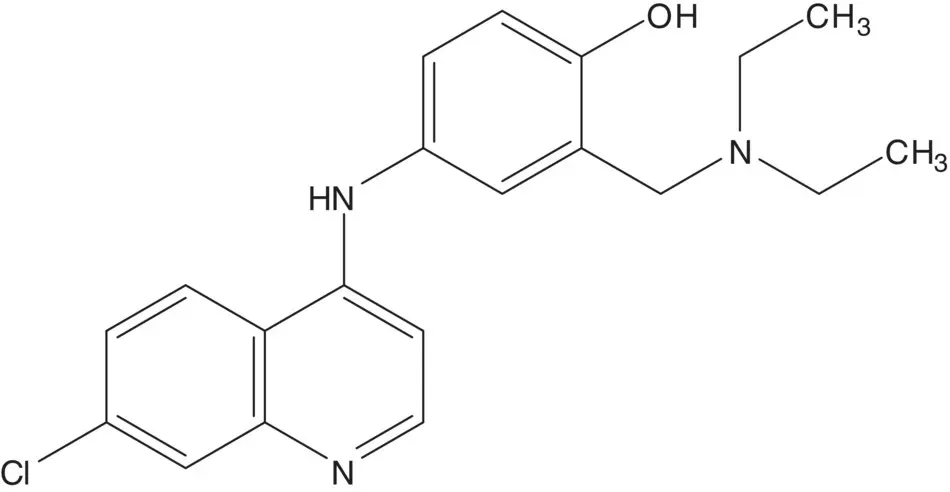
Discussion.A C─N bond is formed by displacement of a chloride at the quinoline 4‐position by nitrogen of the 4‐aminophenol.
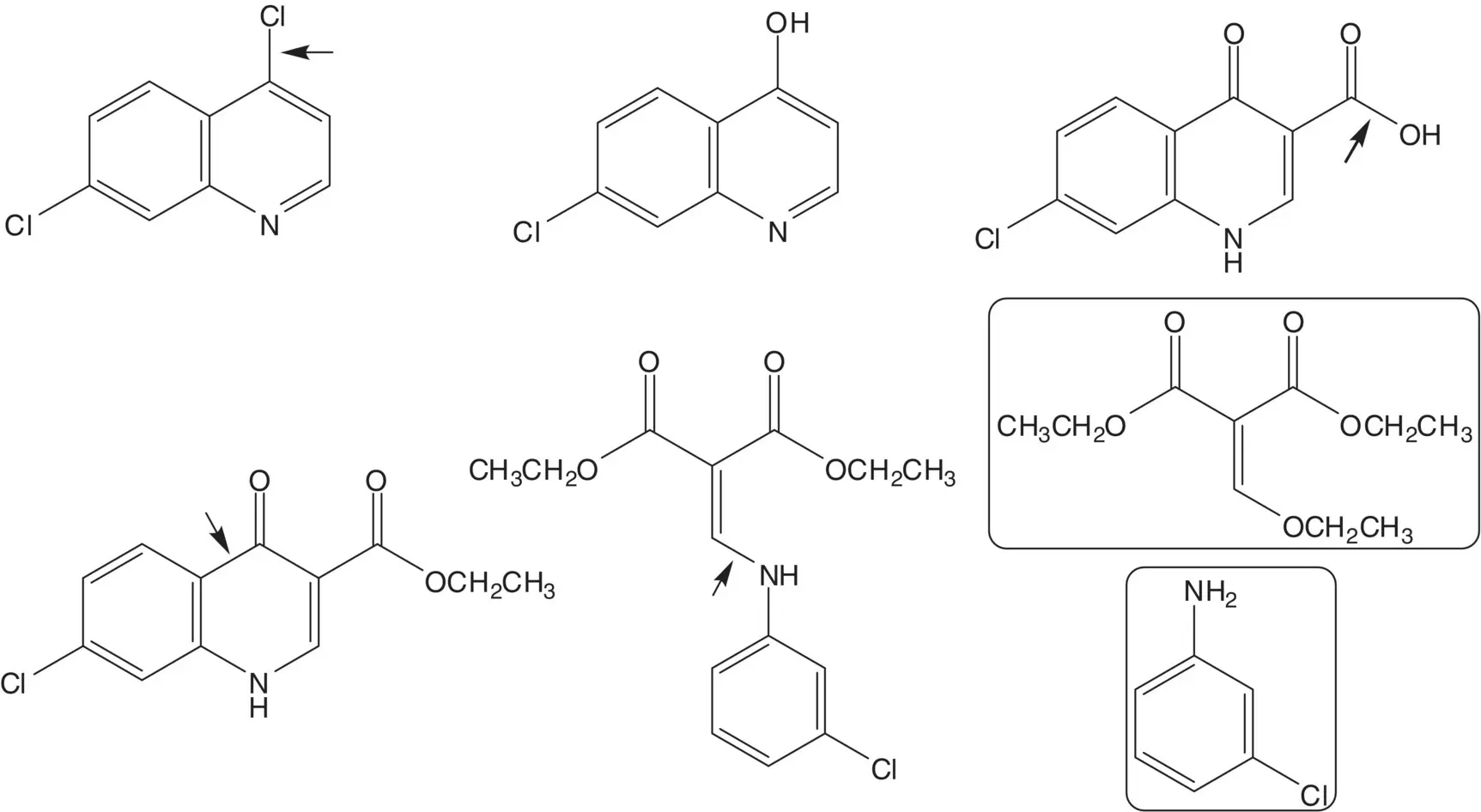
4,7‐Dichloroquinoline is formed from 7‐chloro‐ 4‐hydroxyquinoline (7‐chloroquinolin‐4‐one). 7‐Chloro‐ 4‐hydroxyquinoline is formed by thermolysis/decarboxylation of the 4‐hydroxyquinoline‐3‐carboxylic acid. The carboxylic acid is formed by ester hydrolysis. The quinoline ring is formed by an intramolecular acylation at C6 of the 3‐chloroaniline. An enamine is formed by reaction of the enol ether of ethyl ethoxymethylenemalonate with 3‐chloroaniline (The four‐step sequence from 3‐chloroaniline to 7‐chloro‐4‐hydroxyquinoline is an example of the Gould–Jacobs Reaction).
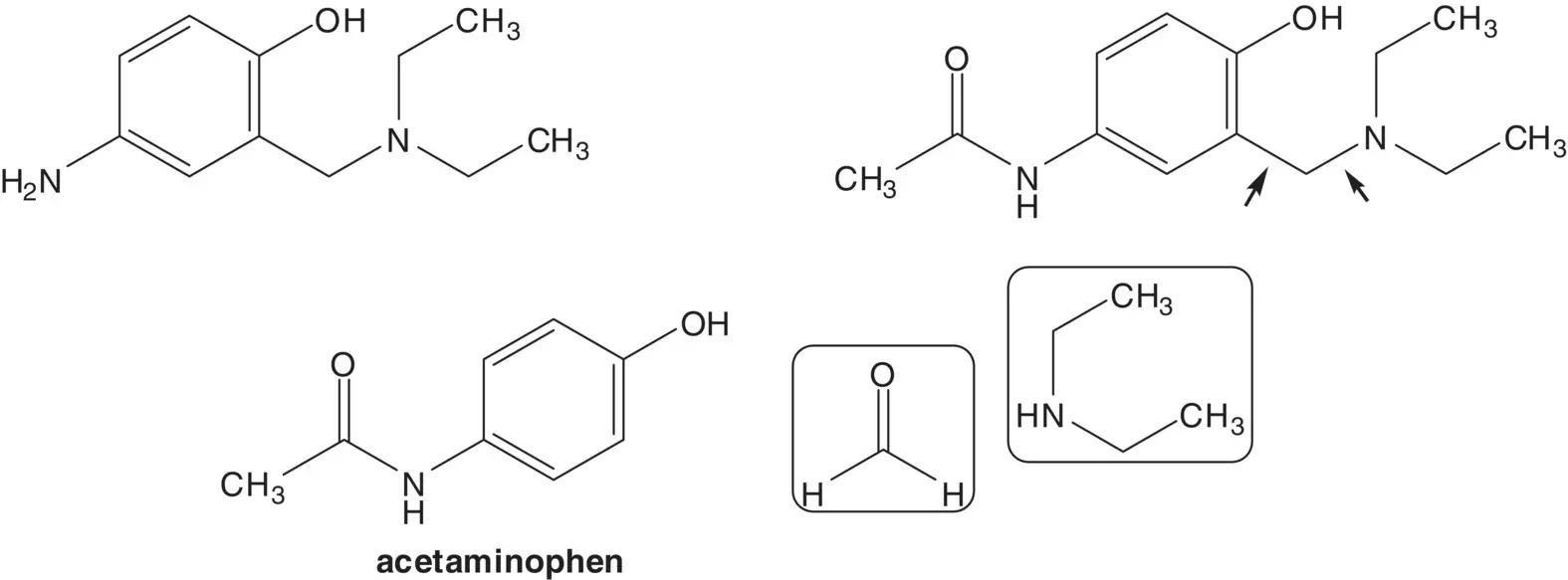
The 4‐aminophenol is formed by hydrolysis of the acetanilide. Another essential medicine, 4‐acetamidophenol ( para ‐acetamol or acetaminophen) is alkylated by reaction with formaldehyde and N,N ‐diethylamine ( Betti Reaction).
Draw the structures of the retrosynthetic analysis of an alternative route from 3‐chloroaniline to 7‐chloro‐4‐hydroxyquinoline utilizing a Conrad–Limpach Reaction. Include the structures of the retrosynthetic analysis of any organic starting material(s) from petrochemical or biochemical raw materials. List pros and cons for the two routes and select one route as the preferred route.
Anti‐Infective Medicines/Antibacterials/Beta‐Lactam Medicines

Penicillins are produced by fermentation or are semisynthetic. A semisynthetic penicillin is often formed by acylation of the amine of 6‐aminopenicillanic acid (6‐APA). 6‐APA is produced from penicillin G (benzylpenicillin) by enzyme‐mediated hydrolysis of the side‐chain amide.
Discussion.Amoxicillin is a semisynthetic penicillin. The final step is enzyme‐mediated formation of the side‐chain amide by reaction of an amine with an ester. The amine, 6‐aminopenicillanic acid (6‐APA), is formed by enzyme‐mediated hydrolysis of the side‐chain amide of penicillin G. Penicillin G (benzylpenicillin) is produced by the fungus Penicillium chrysogenum .
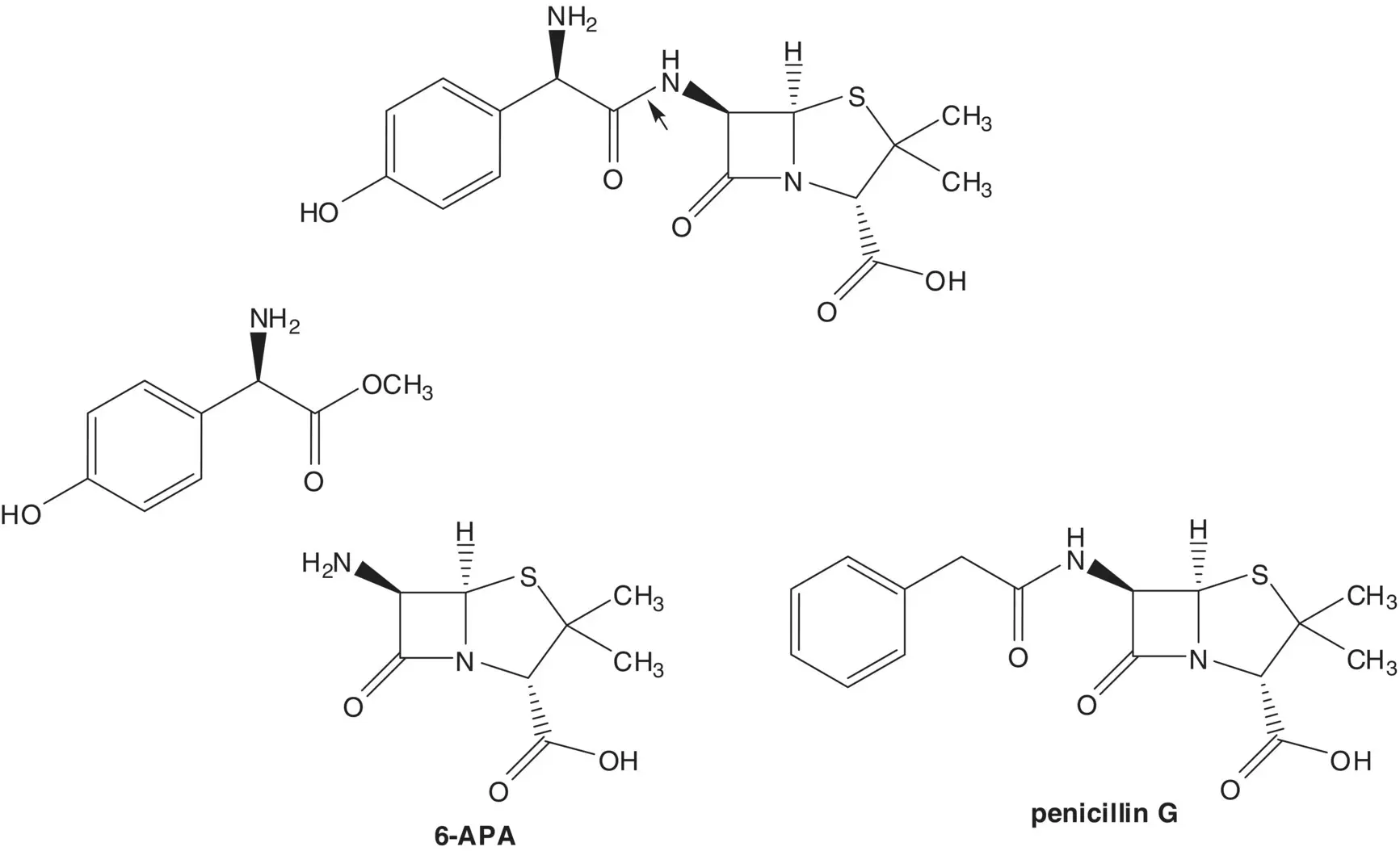
The ester is formed from the carboxylic acid ( Fischer Esterification). The α‐amino acid, ( R )‐α‐(4‐hydroxyphenyl)glycine, is formed by enzyme‐mediated hydrolysis of the N ‐carbamoyl α‐amino acid. The ( R )‐ N ‐carbamoyl α‐amino acid is formed by enzyme‐mediated hydrolysis of the ( R )‐hydantoin in a mixture of the ( R )‐ and ( S )‐hydantoins. Since the ( R )‐ and ( S )‐hydantoins interconvert under the hydrolysis conditions, the ( S )‐hydantoin is also converted to the ( R )‐ N ‐carbamoyl α‐amino acid. The mixture of ( R )‐ and ( S )‐hydantoins is formed from phenol, glyoxylic acid, and urea.
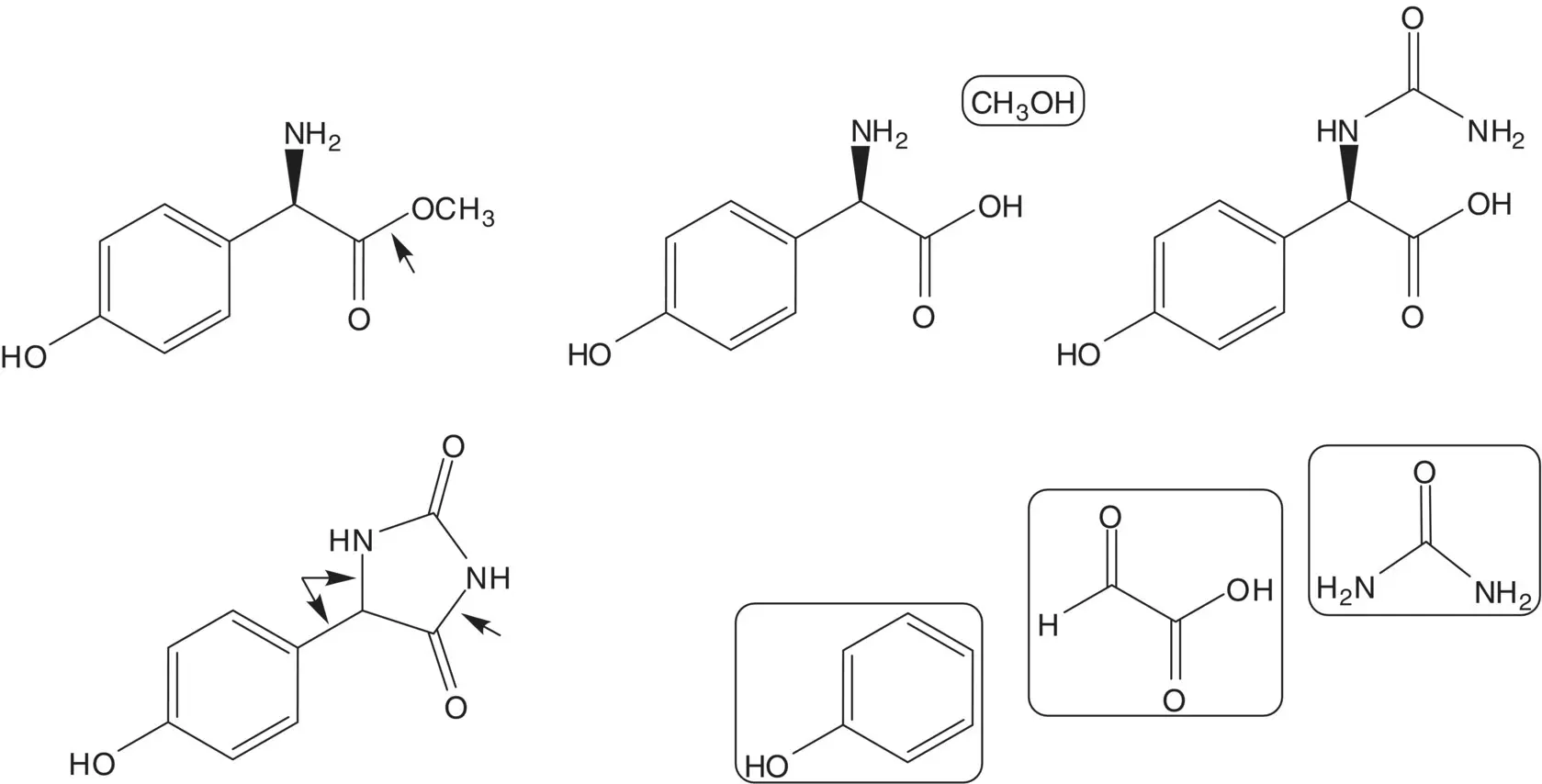
Draw the structures of the retrosynthetic analysis of an alternative route to amoxicillin from ( R )‐α‐(4‐hydroxyphenyl)glycine and 6‐APA which does not utilize an enzyme to mediate the formation of the side‐chain amide bond.
Читать дальше























