A course based on this workbook could be organized in many ways: medicines for pain, medicines containing fluorine, medicines made using a Diels–Alder Reaction, etc. Course development will be facilitated by the provided Indices(name reactions, starting materials from the chiral pool, diazonium salt reactions, epoxide ring‐opening reactions, fluorine‐containing target molecules, furans, imidazoles, nucleophilic aromatic substitution reactions, oxidations, peptides, photochemistry, purines, pyrazines, pyridines, pyrimidines, quinolines, symmetrical molecules, thiazoles, thioethers, triazoles.)
Appendices A, B, and Cand a complete list of the Referencesused in preparation of the workbook are available on the companion website.
www.wiley.com/go/Harrington/routes_essential_medicine
Antiviral Medicines/Antiretrovirals/Nucleoside or Nucleotide Reverse Transcriptase Inhibitors
When two chiral carbons are separated by one or more atoms, disconnections often lead back to an intermediate with the two chiral carbons next to each other and formed in the same reaction.
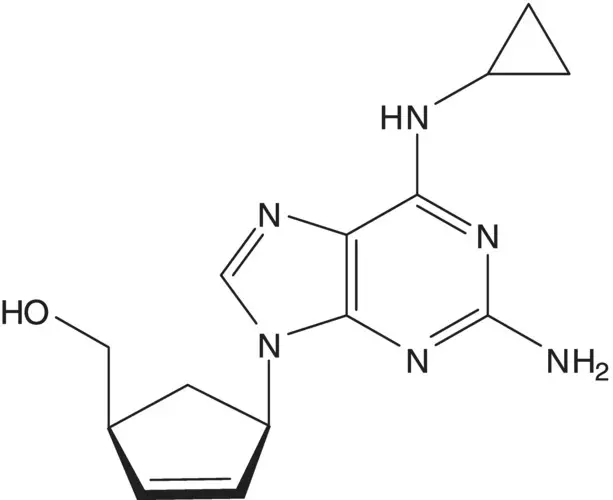
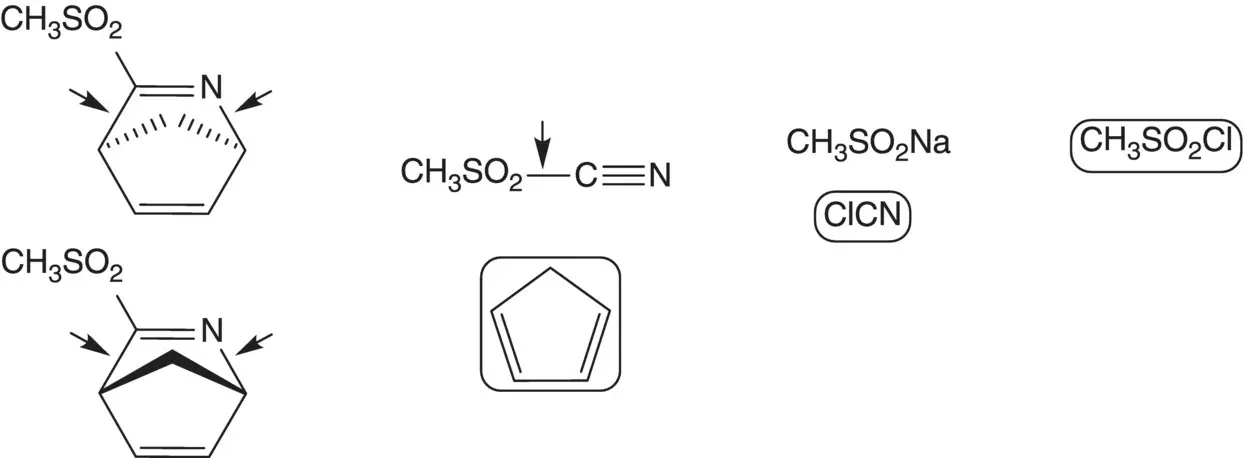
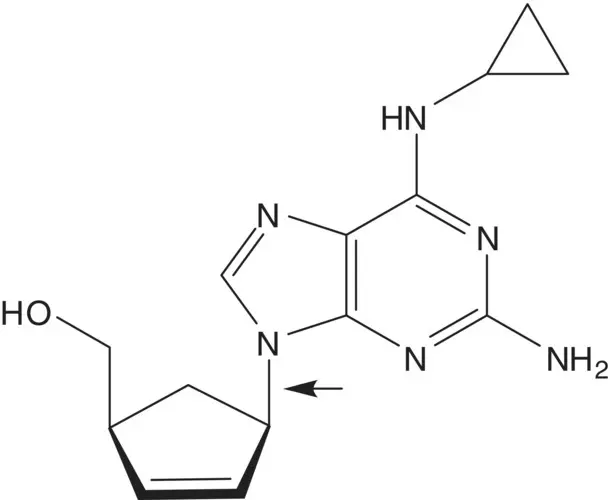
Discussion.Cyclopropanamine is introduced by chloride displacement in the final step (the chloropurine partner is also used to make the AIDS drug carbovir.) The imidazole ring of the purine is formed from the formamide ( Traube Purine Synthesis). A C─N bond is formed by displacement of chloride from the symmetrical dichloropyrimidine by the amine of (1 S ,4 R )‐4‐amino‐2‐cyclopentene‐1‐methanol.
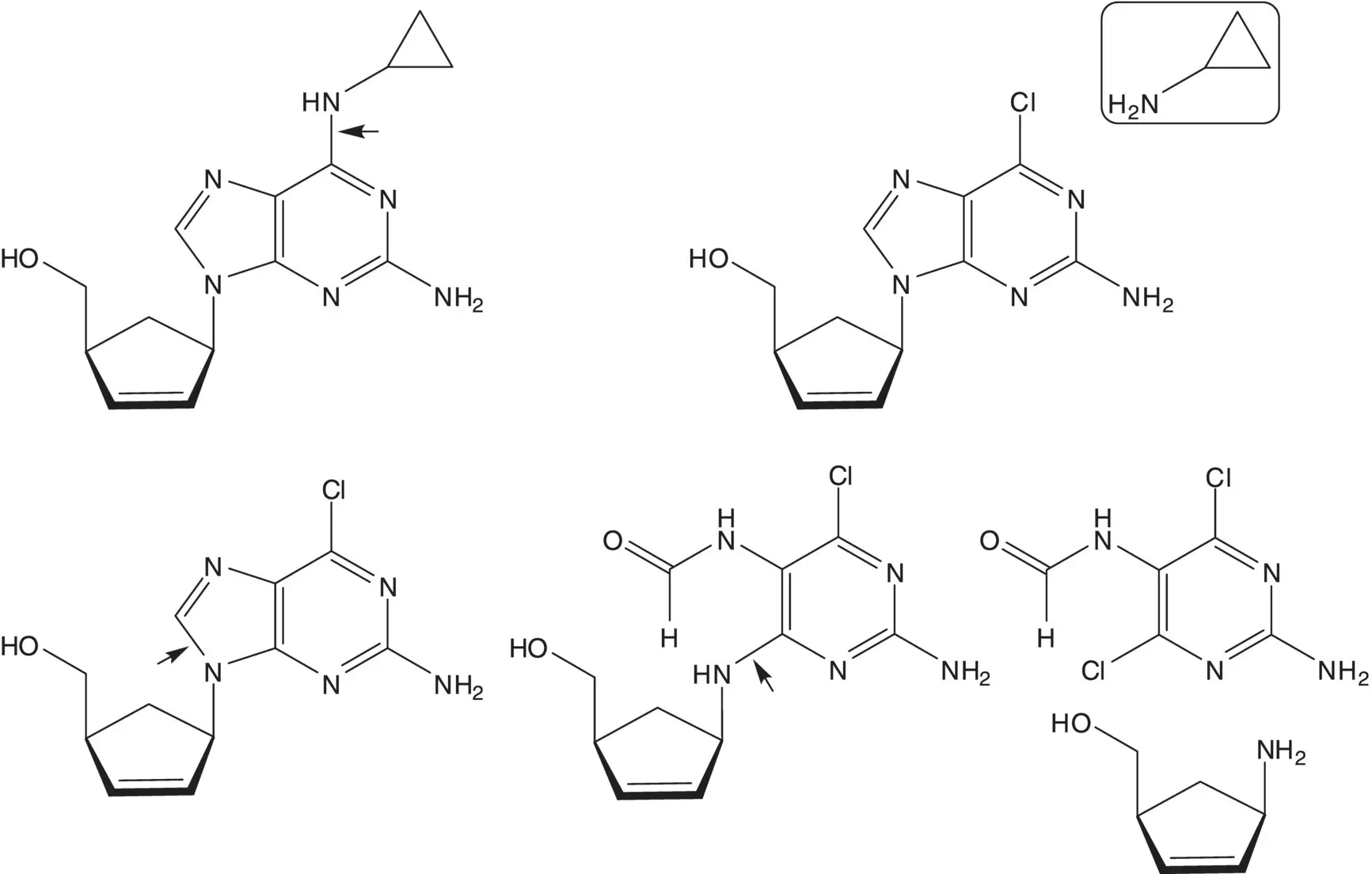
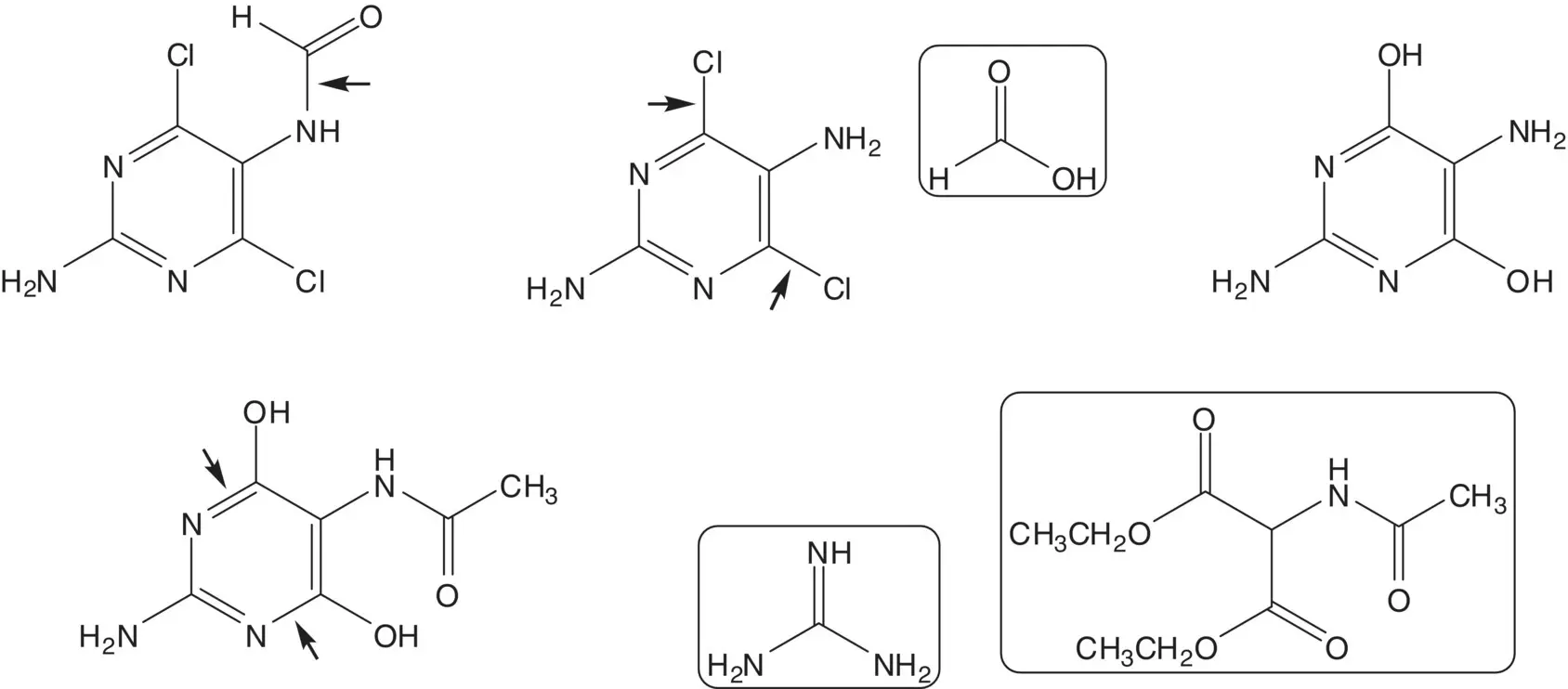
The 2,5‐diaminopyrimidine 5‐formamide is formed from the 2,5‐diaminopyrimidine and formic acid. 2,5‐Diamino‐4,6‐dichloropyrimidine is formed from 2,5‐diamino‐4,6‐dihydroxypyrimidine. 2,5‐Diamino‐4,6‐dihydroxypyrimidine is formed by hydrolysis of the 5‐acetamide. 5‐Acetamido‐2‐amino‐4,6‐dihydroxypyrimidine ring is formed from guanidine and diethyl acetamidomalonate ( Pinner Pyrimidine Synthesis).
(1 S ,4 R )‐4‐Amino‐2‐cyclopentene‐1‐methanol and the (1 R ,4 S )‐enantiomer are separated by resolution. The amino alcohols are formed by reduction of the amides ( Vince Lactam). The amides are formed by displacement of methanesulfinate by hydroxide.
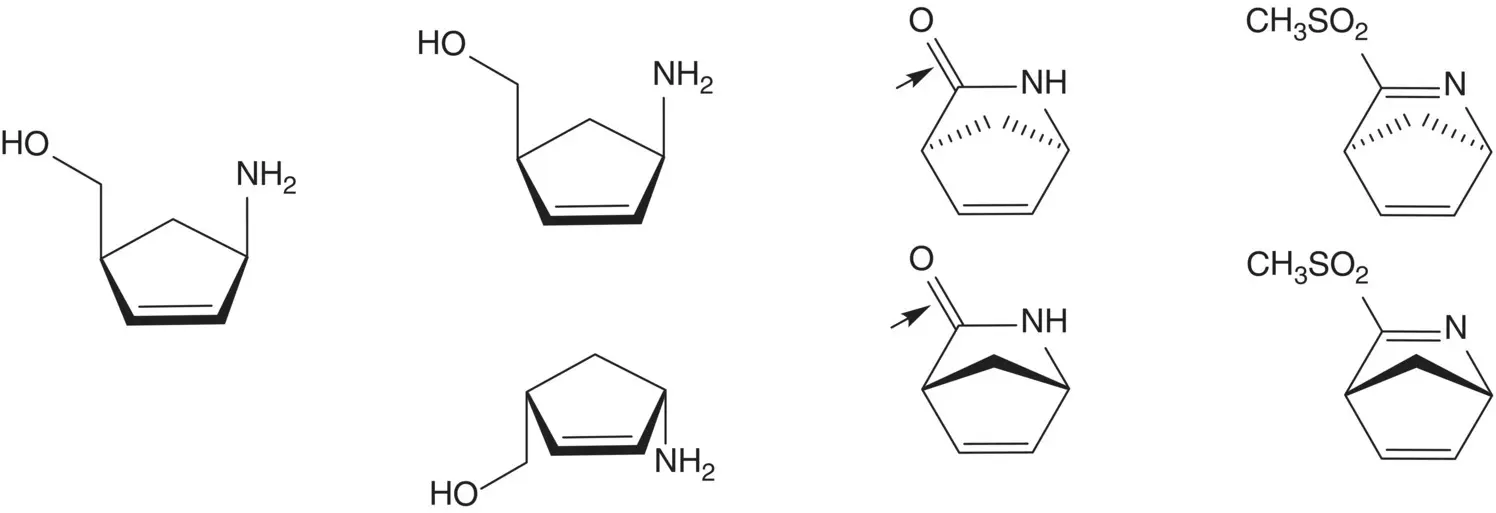
The 2‐azabicyclo[2.2.1]hepta‐2,5‐dienes are formed by [4 + 2]‐cycloaddition of cyclopentadiene with methanesulfonyl cyanide ( Diels–Alder Cycloaddition). Methanesulfonyl cyanide is formed from sodium methanesulfinate and cyanogen chloride. Sodium methanesulfinate is formed by reduction of methanesulfonyl chloride.
Draw the structures of the retrosynthetic analysis of one alternative route to abacavir using a disconnection of the C─N bond joining the purine ring to the cyclopentene ring. Include the structures of the retrosynthetic analysis of any organic starting material(s) from petrochemical or biochemical raw materials.
Ophthalmological Preparations/Miotics and Antiglaucoma Medicines
A sulfonyl chloride attached to an aromatic ring may be formed by oxidation of the thiol.
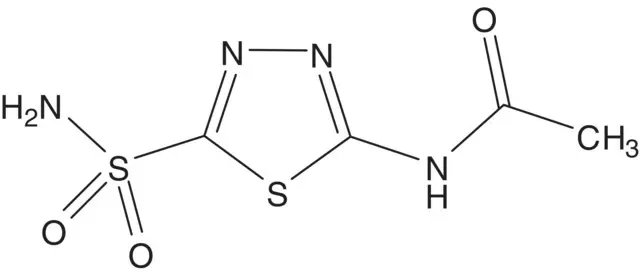
Discussion.Acetazolamide is formed in just three steps from 5‐amino‐1,3,4‐thiadiazole‐2‐thiol. The sulfonamide is formed from the sulfonyl chloride in the final step. The sulfonyl chloride is formed by oxidation of the thiol. The amide is formed by acetylation of the amine using acetic anhydride.
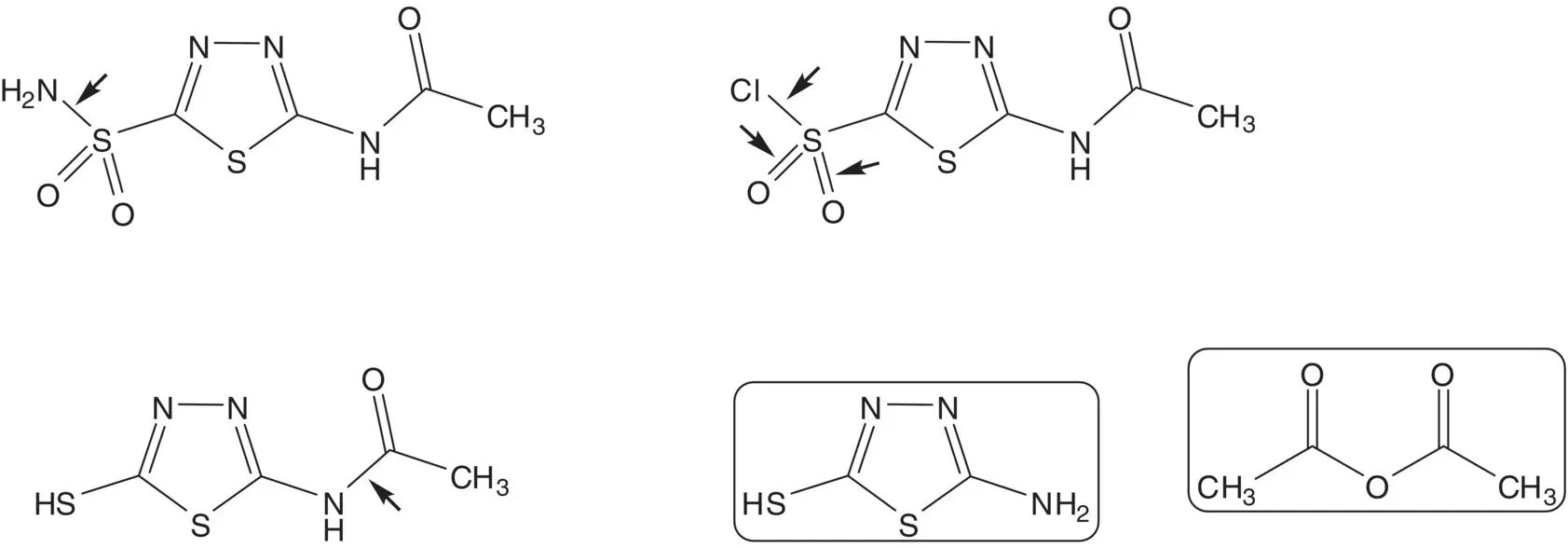
Draw the structures of the retrosynthetic analysis of one alternative route to the starting material 5‐amino‐1,3,4‐thiadiazole‐2‐thiol. List the pros for the route presented and the alternative route and select one route as the preferred route.
Antidotes/Specific
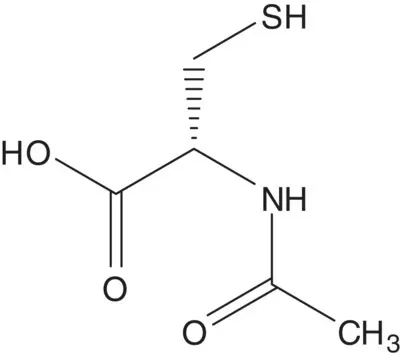
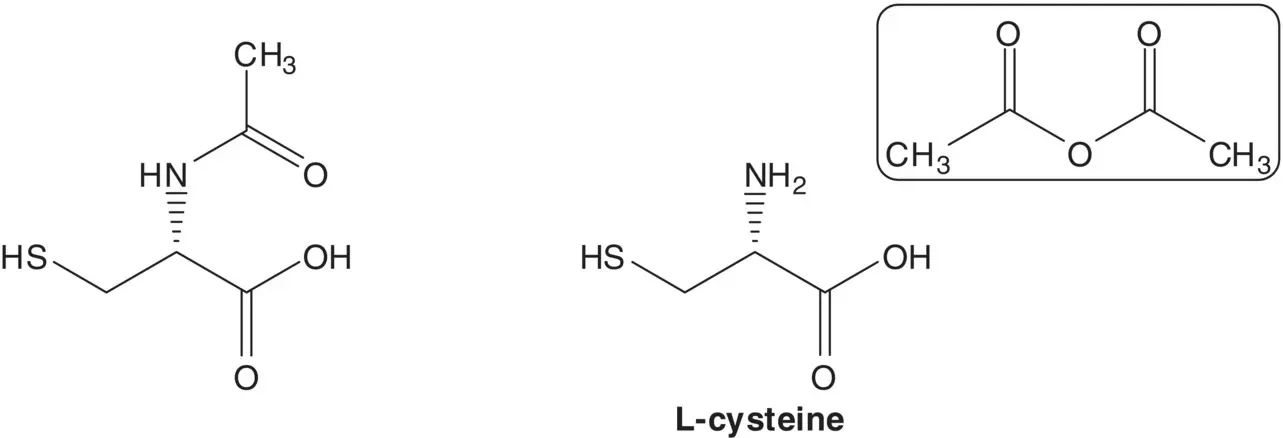
A chiral carbon in a single‐enantiomer molecule is often delivered in a starting material.
Discussion. N ‐Acetyl‐L‐cysteine is formed by acetylation of L‐cysteine with acetic anhydride. L‐Cysteine is produced by fermentation.
Medicines for Pain and Palliative Care/Non‐Opioids and Non‐Steroidal Anti‐Inflammatory Medicines
Antimigraine Medicines/For Treatment of Acute Attack
Cardiovascular Medicines/Antithrombotic Medicines/Anti‐Platelet Medicines
Medicines for Diseases of Joints/Juvenile Joint Diseases
salicylic acid
Dermatological Medicines/Medicines Affecting Skin Differentiation and Proliferation
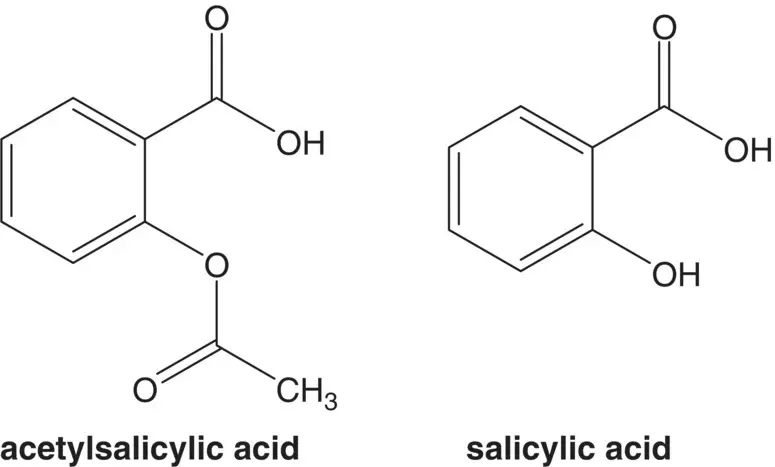
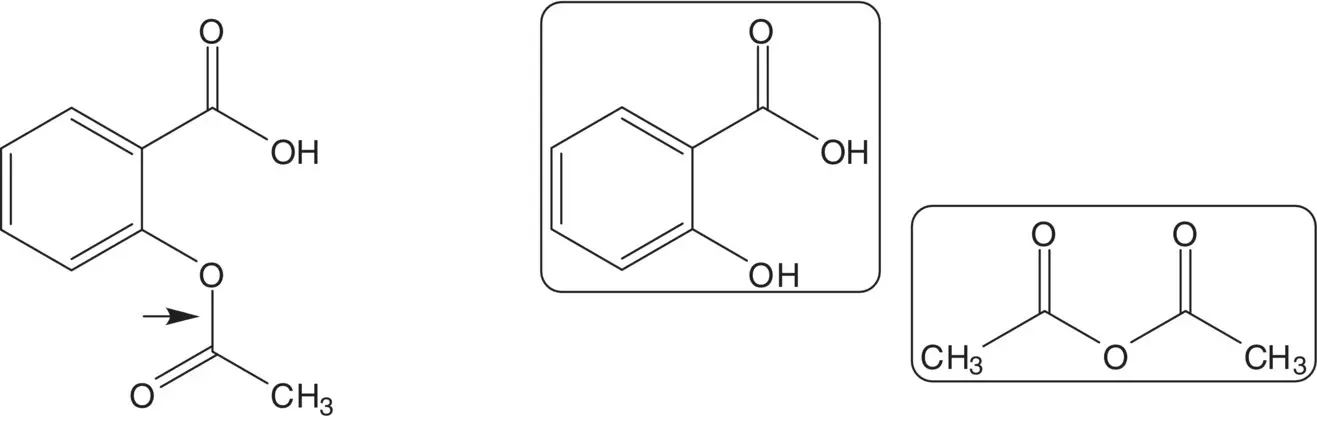
Discussion.Acetylsalicylic acid (aspirin) is formed from another essential medicine, salicylic acid, and acetic anhydride. Salicylic acid is formed from phenol and carbon dioxide ( Kolbe–Schmitt Reaction).
Anti‐Infective Medicines/Antiviral Medicines/Antiherpes Medicines
Ophthalmological Preparations/Anti‐Infective Agents
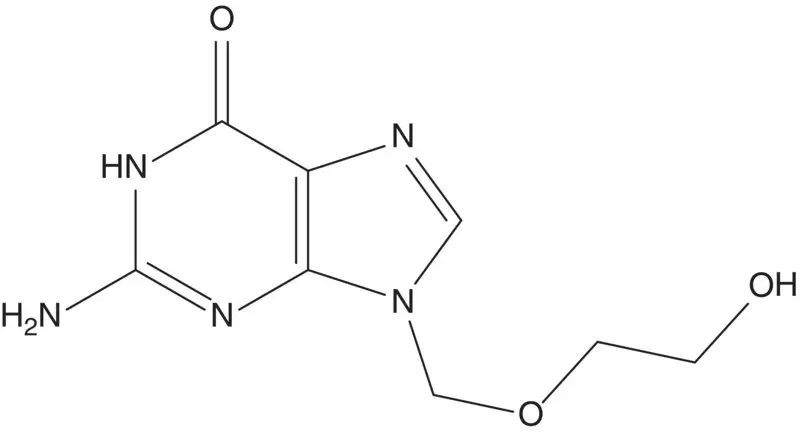
Guanine is often converted to an acylated or silylated derivative to increase solubility in organic solvents. These derivatives react with alkylating agents to form a mixture of N7‐alkylated (kinetic) product and N9‐alkylated (thermodynamic) product.
Читать дальше

























