Discussion.The concepts and challenges common to the many routes to acyclovir are featured in a comparison of two preferred routes. In route A, the alcohol is released by O ‐desilylation in the final step. Acyclovir O ‐trimethylsilyl ether is formed by desilylation of persilyl acyclovir. A mixture of the N9‐alkylated persilyl acyclovir and the N7‐alkylated regioisomer is formed in situ by in the reaction of persilyl guanine with 1,3‐dioxolane (What is the highest ratio of persilyl acyclovir to the N7‐alkylated regioisomer? What reaction conditions are associated with the highest ratio? How is the N7‐alkylated side product separated from the N9‐alkylated product?). Persilyl guanine is a mixture of N7‐TMS and N9‐TMS regioisomers formed in situ by the reaction of guanine with excess hexamethyldisilazane (HMDS).
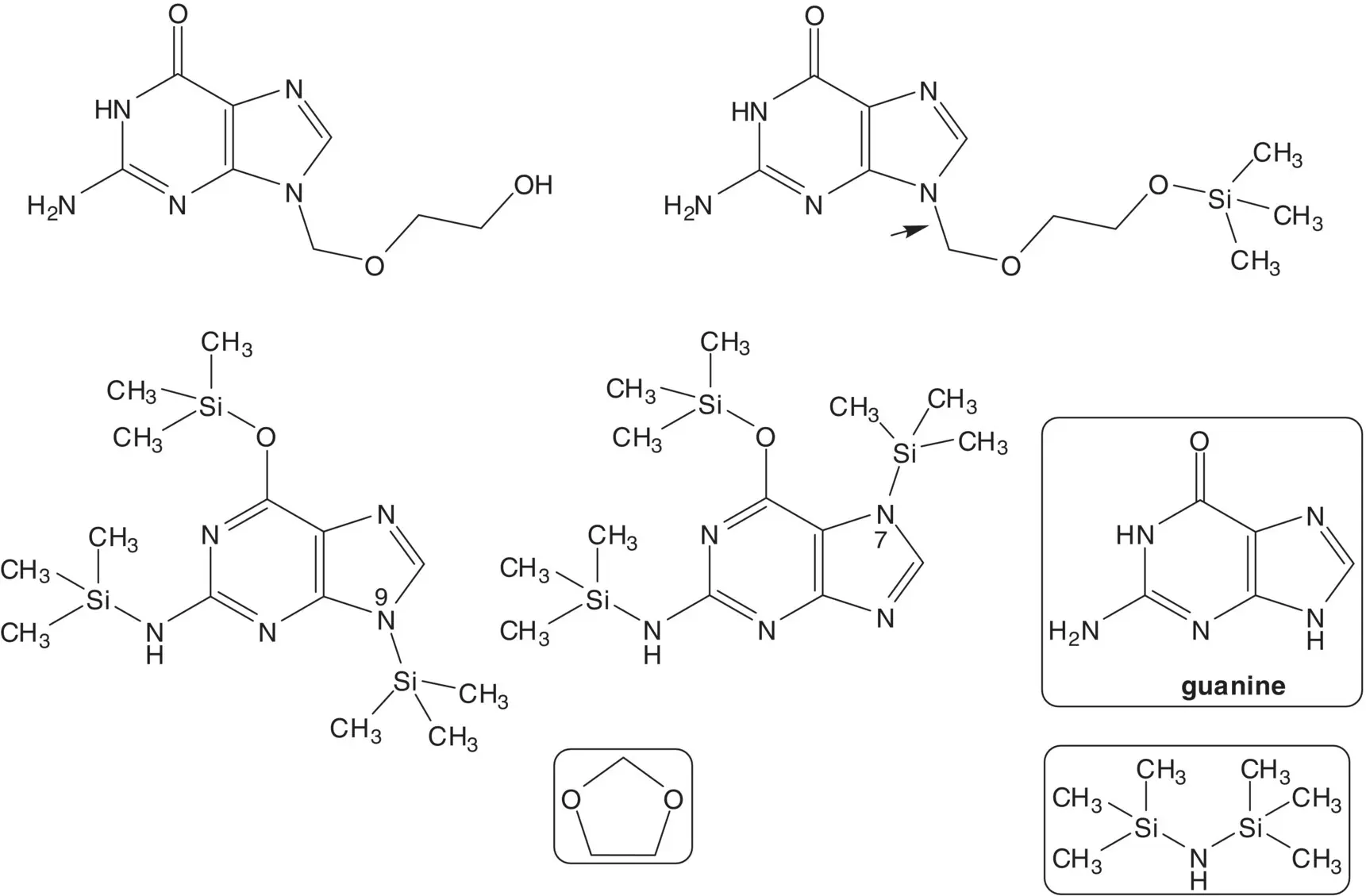
In route B, the alcohol and amino group are released by hydrolysis of the ester and amide in the final step. The alkylation of N2,9‐diacetylguanine with 2‐(acetoxyethoxy)methyl acetate affords a mixture of the N7‐ and N9‐regioisomers. (Draw the structure of the N7‐regioisomer. What is the highest N9:N7 ratio? What reaction conditions are associated with the highest ratio? How is the N7‐alkylated side product separated from N9‐alkylated product?) N2,9‐Diacetylguanine is formed by reaction of guanine with acetic anhydride.
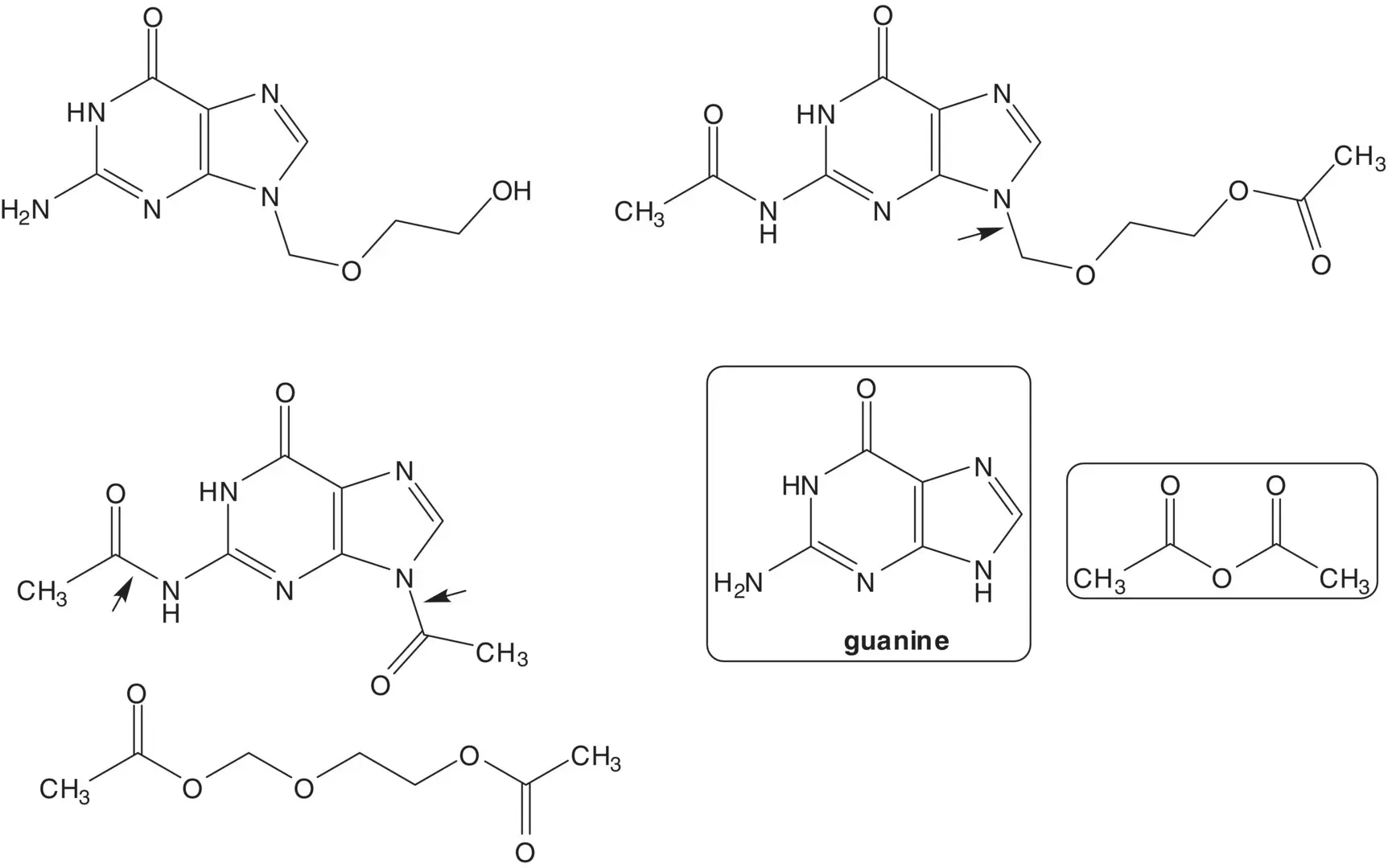
2‐(Acetoxyethoxy)methyl acetate is formed from 1,3‐dioxolane, acetic acid, and acetic anhydride.
List the pros and cons for routes A and B and select one route as the preferred route.
Anti‐Infective Medicines/Anthelmintics/Antifilarials
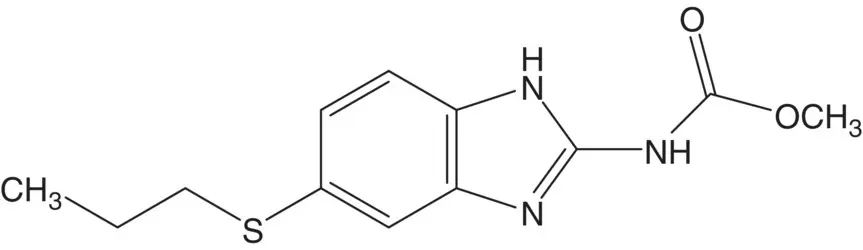
A benzimidazole is often formed from a 1,2‐phenylenediamine.
Discussion.The benzimidazole is formed in the final step from the benzene‐1,2‐diamine and N ‐methoxycarbonylcyanamide. N ‐Methoxycarbonylcyanamide is formed from cyanamide and methyl chloroformate. 4‐(Propylthio)benzene‐1,2‐diamine is formed by reduction of 2‐nitro‐4‐propylthioaniline. A C─S bond is formed by displacement of chloride from 4‐chloro‐2‐nitroacetanilide by sodium propanethiolate. The acetanilide is also hydrolyzed under the chloride displacement reaction conditions. 4‐Chloro‐2‐nitroacetanilide is formed from 4‐chloro‐2‐nitroaniline and acetic anhydride.
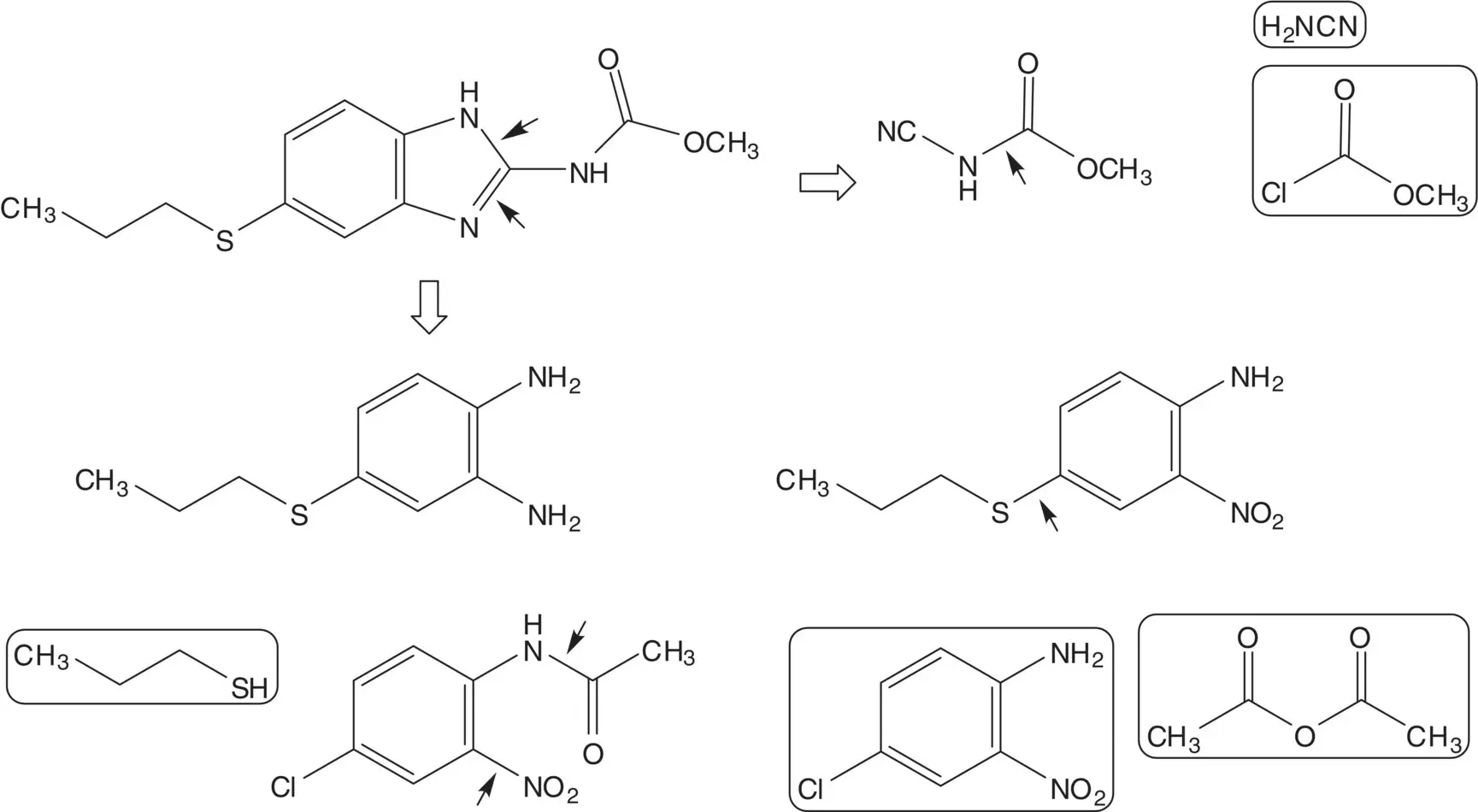
2‐Nitro‐4‐propylthioaniline can also be manufactured from 1‐chloro‐2‐nitrobenzene. Draw the structures of the retrosynthetic analysis of this route. List the pros and cons for both routes. Which route is preferred?
Antineoplastics and Immunosuppressives/Cytotoxic and Adjuvant Medicines
Medicines for Diseases of Joints/Medicines Used to Treat Gout
Hydrazine is often the source of the two nitrogen atoms in a pyrazole ring.
Discussion.The pyrimidine ring of the pyrazolo[3,4‐d]pyrimidine is formed in the final step by reaction of 3‐aminopyrazole‐4‐carboxamide with formamide. The pyrazole ring is formed from 2‐cyano‐3‐morpholinoacrylamide and hydrazine. The enamine of 2‐cyano‐3‐morpholinoacrylamide is formed from the enol ether by the displacement of ethanol by morpholine. The enol ether of 2‐cyano‐3‐ethoxyacrylamide is formed by the reaction of 2‐cyanoacetamide with triethyl orthoformate.
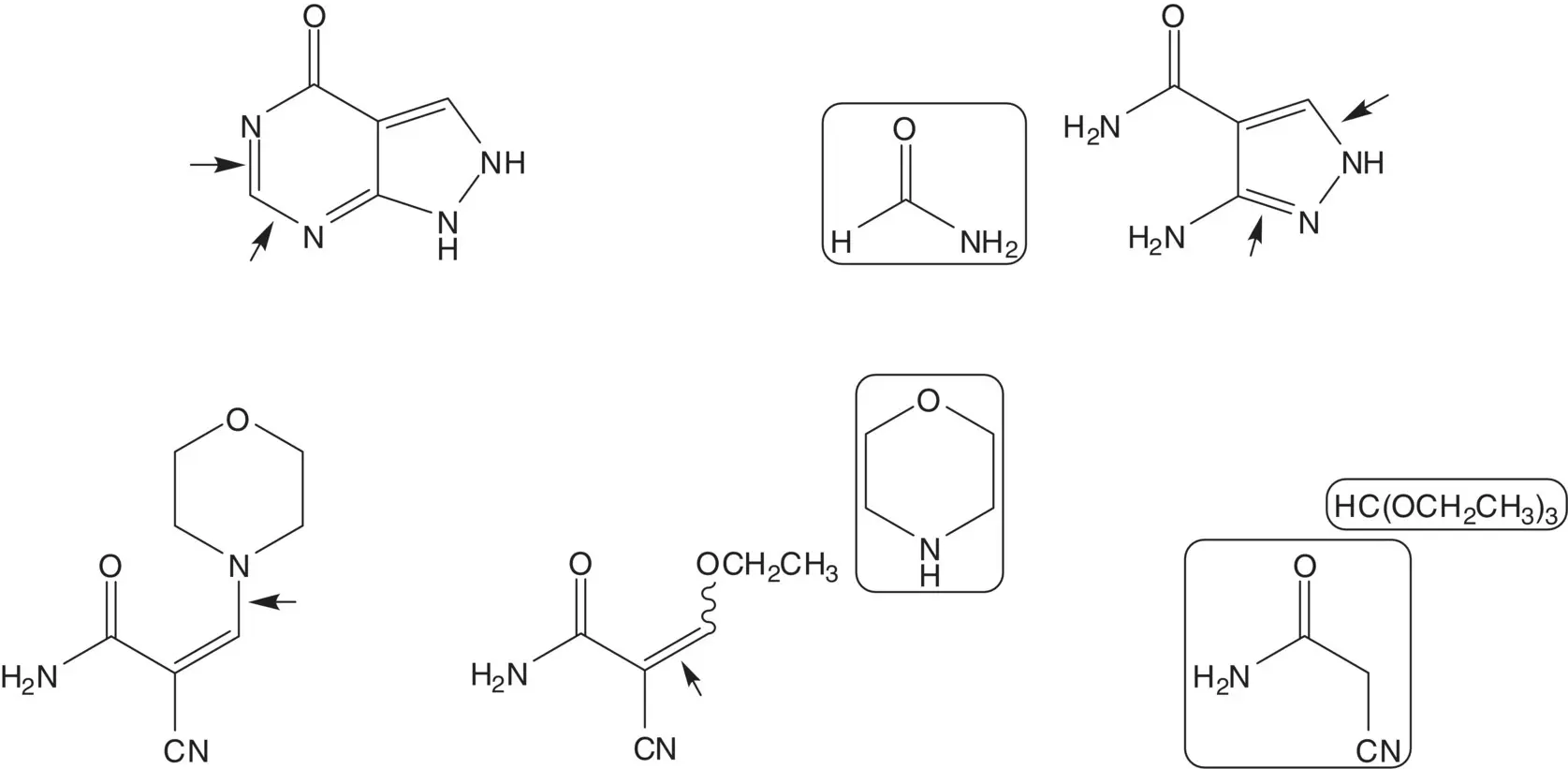
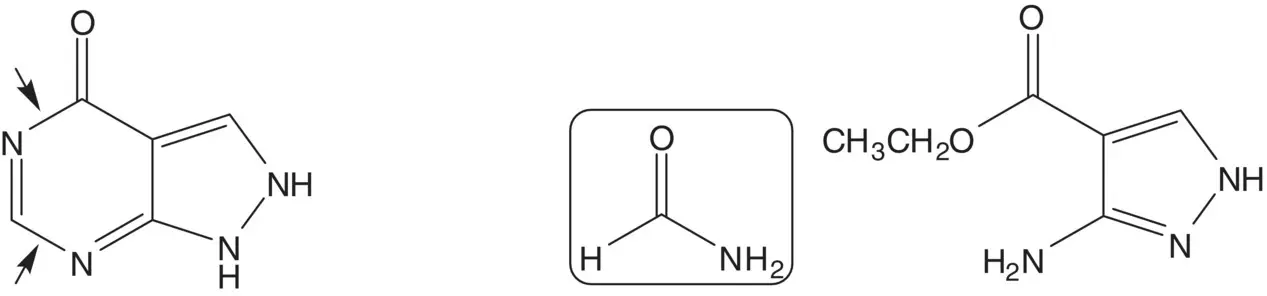
The pyrimidine ring is also formed by reaction of ethyl 3‐aminopyrazole‐4‐carboxylate with formamide. Draw the structures of the retrosynthetic analysis of ethyl 3‐aminopyrazole‐4‐carboxylate. List the pros and cons for both routes and select one route as the preferred route.
Diagnostic Agents/Radiocontrast Media
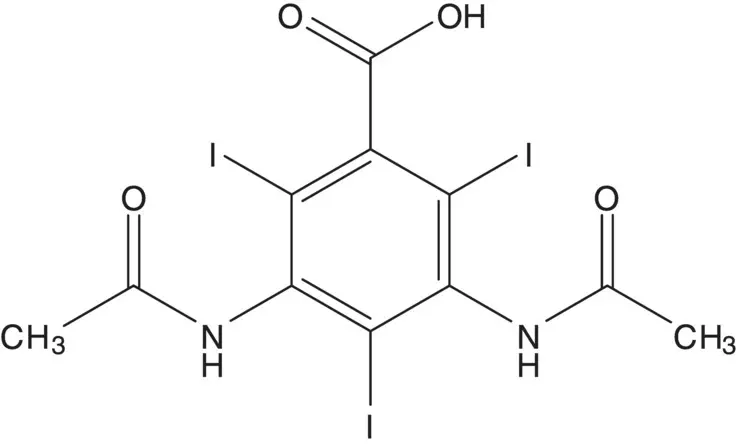
For a symmetrical molecule, symmetrical disconnections lead back to symmetrical intermediates and are likely associated with the shortest route.
Discussion.Since some amide hydrolysis is likely under iodination conditions, the diamide is formed in the final step by reaction of the diamine with acetic anhydride. The triiodide is formed by iodination of 3,5‐diaminobenzoic acid.
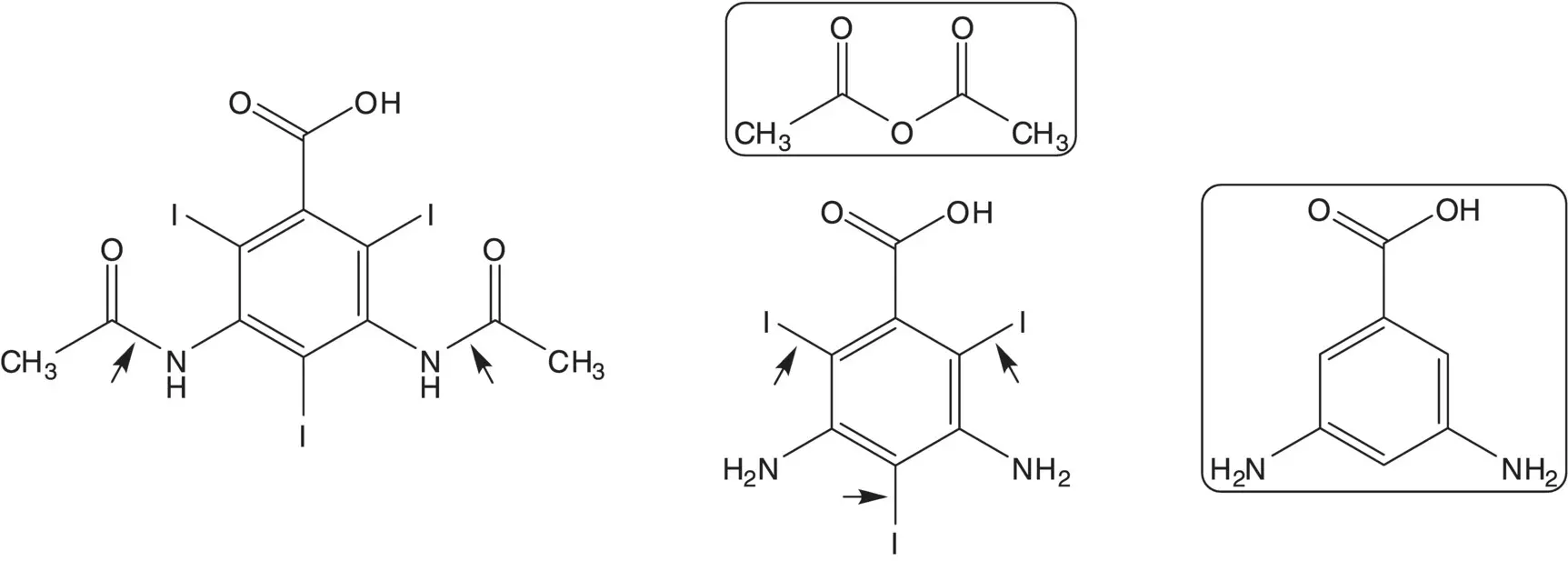
List reagents or reagent combinations used for direct iodination of an aromatic ring.
Anti‐Infective Medicines/Antibacterials/Other Antibacterials
Anti‐Infective Medicines/Antibacterials/Antituberculosis Medicines
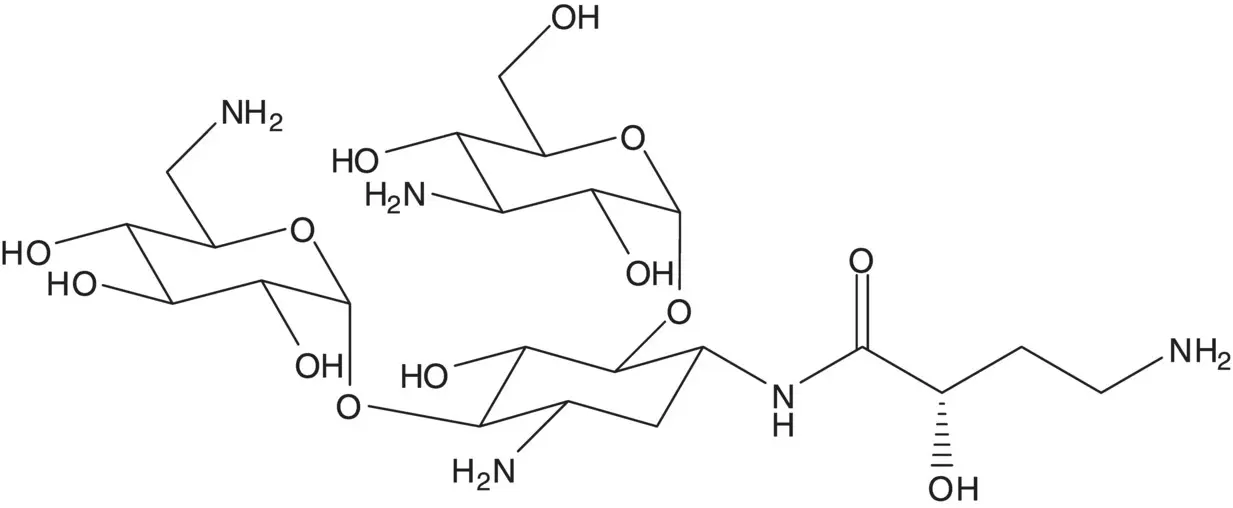
A single‐enantiomer molecule with multiple chiral carbons is often made by modification of a natural product which has most or all of the chiral carbons already in place.
Discussion.Amikacin is semisynthetic. Amikacin is formed by acylation of the amino group at C1 of kanamycin A. This selective acylation requires a protection–deprotection strategy since kanamycin A has four amino groups and the amino group at C1 is not the most reactive.
Three of the amino groups of amikacin are released in the final step by benzyl carbamate hydrogenolysis. The amide at C1 is formed by reaction of the amino group with an N ‐hydroxysuccinimide ester. Amino groups at C3 and C6′ of kanamycin A are protected as benzyl carbamates (Cbz). Kanamycin A is produced by fermentation.
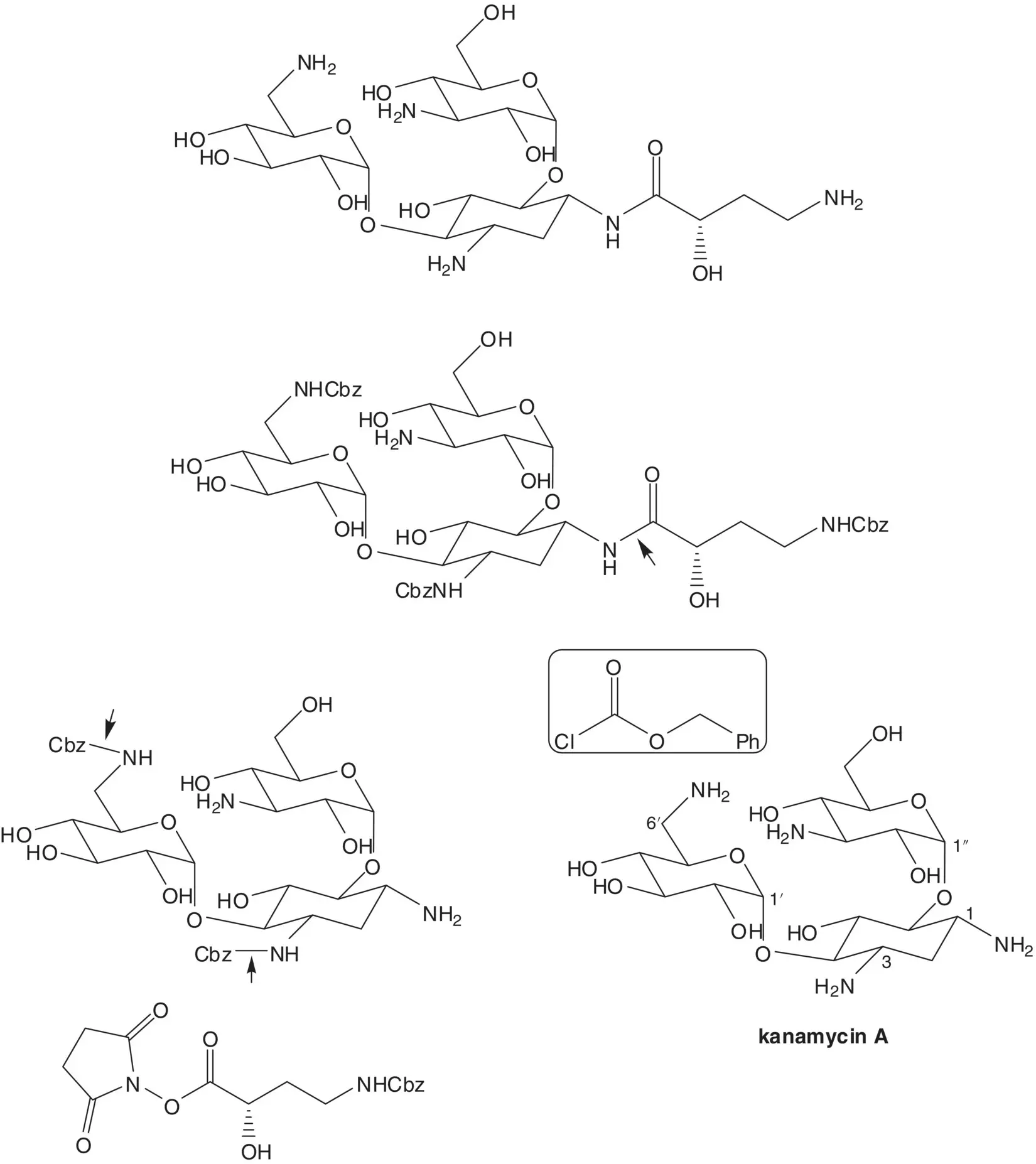
The N ‐hydroxysuccinimide ester is formed from the carboxylic acid. The amino group of the 4‐amino‐2‐hydroxybutanoic acid is protected as the benzyl carbamate. ( S )‐4‐Amino‐2‐hydroxybutanoic acid is formed from (S)‐2‐hydroxyglutaramic acid ( Hofmann Rearrangement). The amide is formed from the lactone. ( S )‐5‐Oxotetrahydrofuran‐2‐carboxylic acid lactone is formed by diazotization of L‐glutamic acid. L‐Glutamic acid is produced by fermentation.
Читать дальше
























