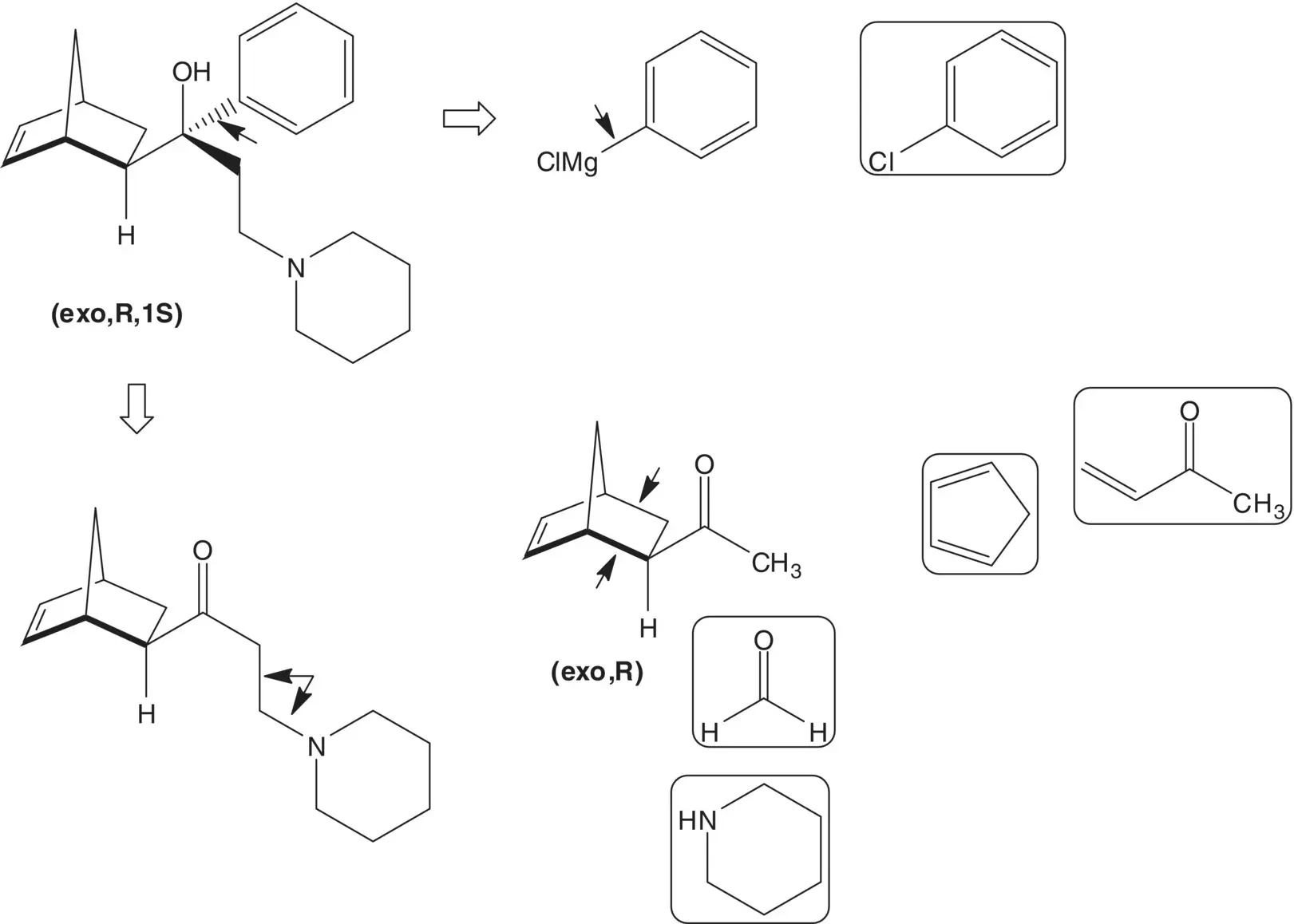
Draw the structures of the retrosynthetic analysis of one alternative route to biperiden. List the pros and cons for both routes and select one route as the preferred route.
Cardiovascular Medicines/Antiarrhythmic Medicines
Cardiovascular Medicines/Medicines Used in Heart Failure
A β–amino alcohol is often formed by ring‐opening an epoxide with an amine.
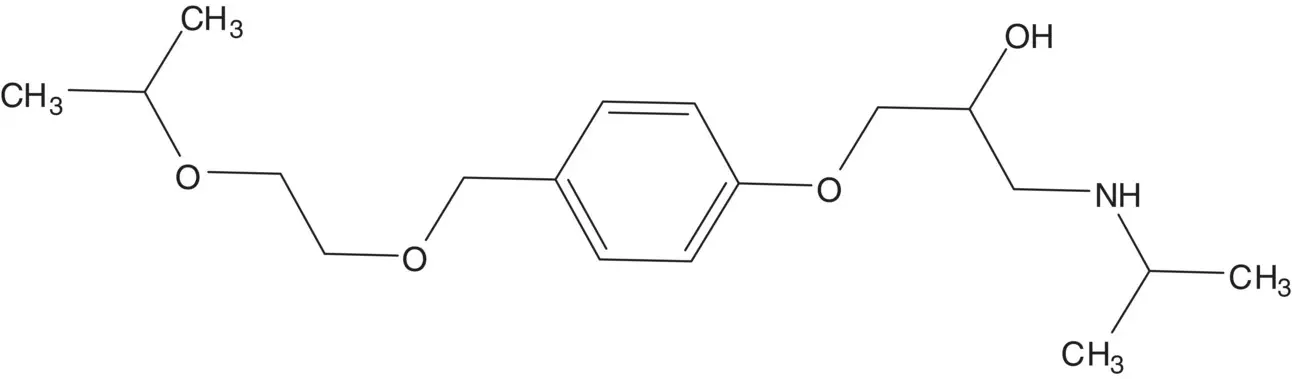
Discussion.Bisoprolol is a 1 : 1 mixture of ( R )‐ and ( S )‐enantiomers. The β–amino alcohol is formed in the final step by ring‐opening of the epoxide with isopropylamine. The epoxide is formed from the chlorohydrin. The chlorohydrin is formed by the ring‐opening of epichlorohydrin by the phenol. The benzyl ether is formed by the reaction of 4‐hydroxybenzyl alcohol with 2‐isopropoxyethanol.
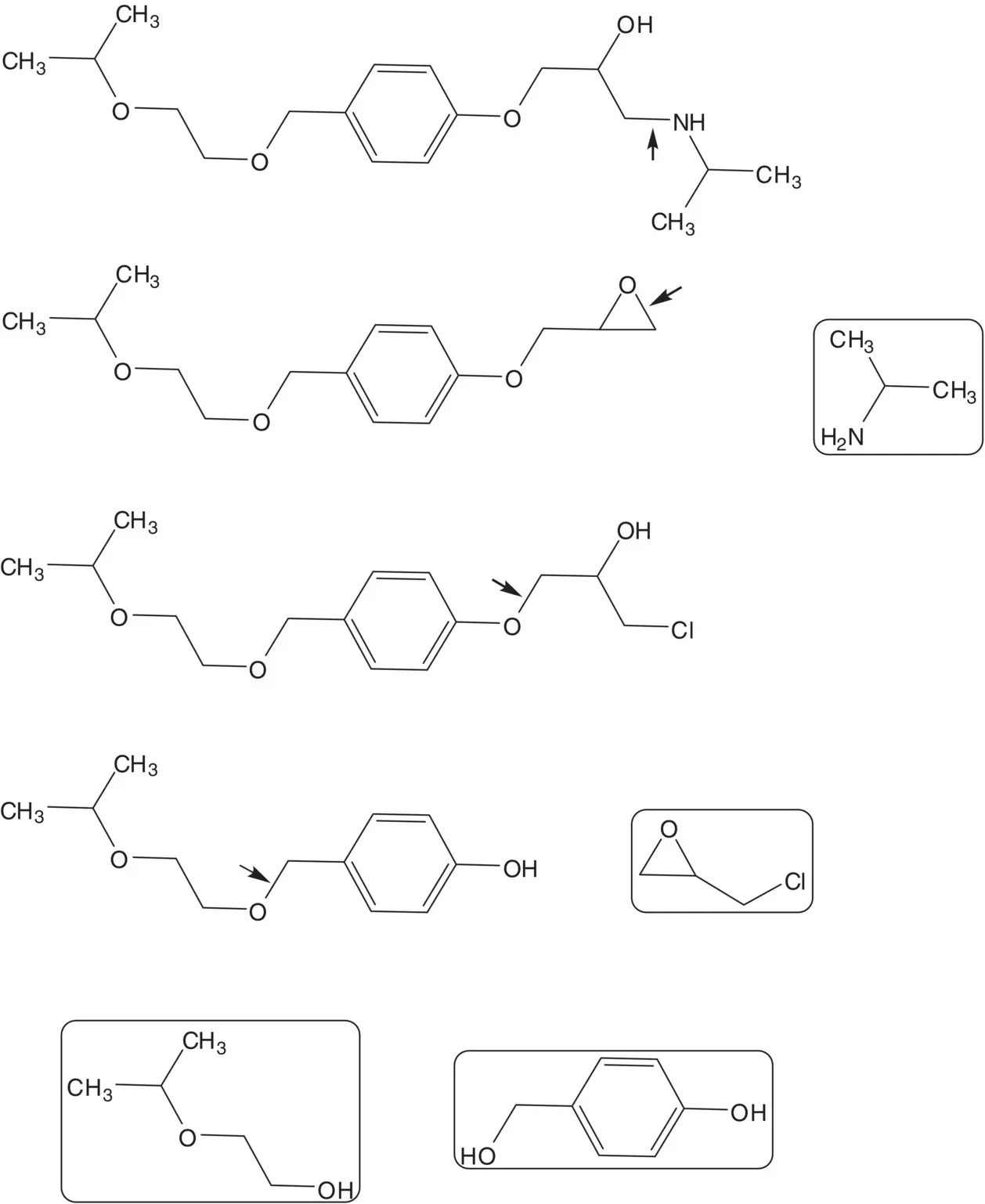
Draw the structures of the retrosynthetic analysis of one alternative route to bisoprolol from phenol that does not have 4‐hydroxybenzaldehyde as an intermediate. List the pros and cons for both routes. Is one route preferred?
Medicines Acting on the Respiratory Tract/Antiasthmatic and Medicines for Chronic Obstructive Pulmonary Disease
Ear, Nose, and Throat Medicines
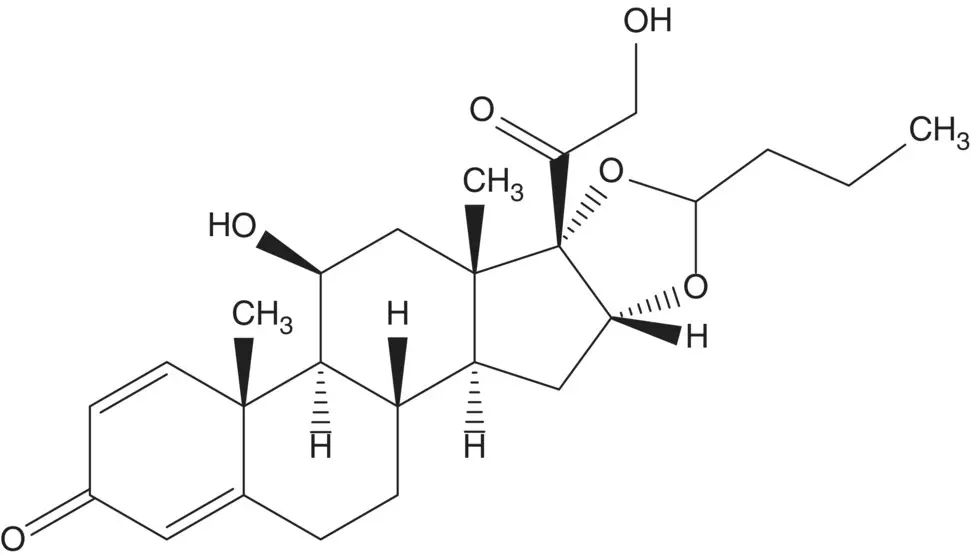
A single‐enantiomer molecule with multiple chiral carbons is often formed by modification of a natural product which has most or all of the chiral carbons already in place. A 16α,17α‐dihydroxy‐20‐one steroid is often formed by dihydroxylation of a 16‐ene‐20‐one.
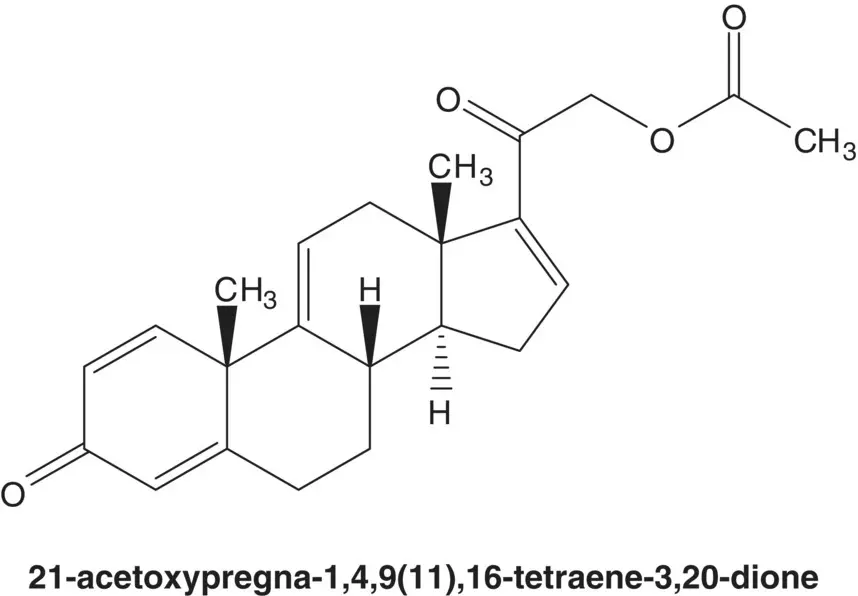
Discussion.Budesonide is manufactured in five steps from 21‐acetoxypregna‐1,4,9(11),16‐tetraene‐3,20‐dione and in 10 steps from another essential medicine, prednisolone.
Budesonide is a mixture of the 22 R ‐ and 22 S ‐diastereomers. The acetal is formed in the final step by reaction of the 16α,17α‐diol with butanal. The C21 alcohol is released by hydrolysis of the acetate ester. The 9α‐hydrogen is introduced by reduction of the 9α‐bromide. The bromohydrin is formed from the 9(11)‐ene. The 16α,17α‐diol is formed by dihydroxylation of the 16‐alkene of 21‐acetoxypregna‐1,4,9(11),16‐tetraene‐3,20‐dione.
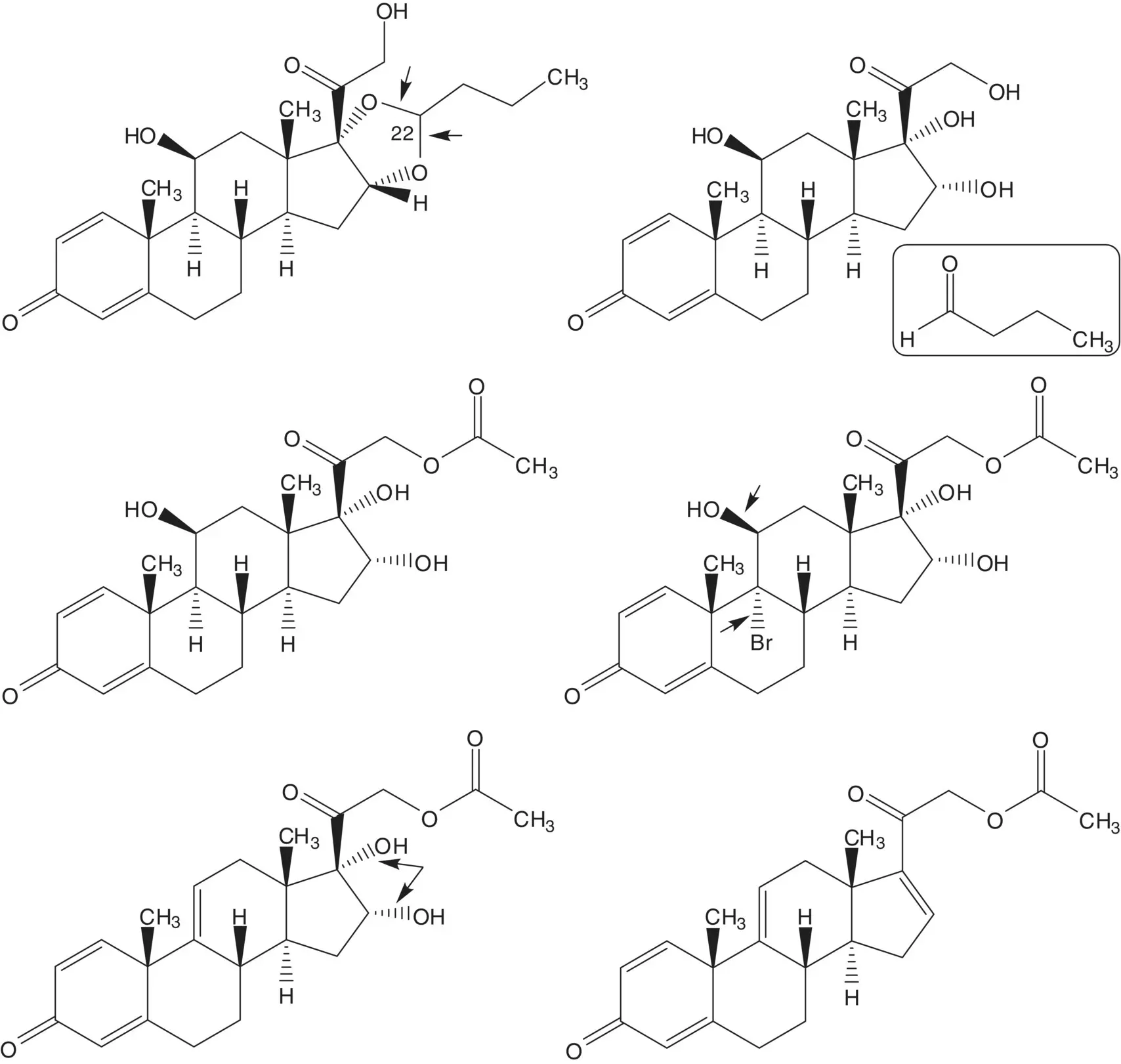
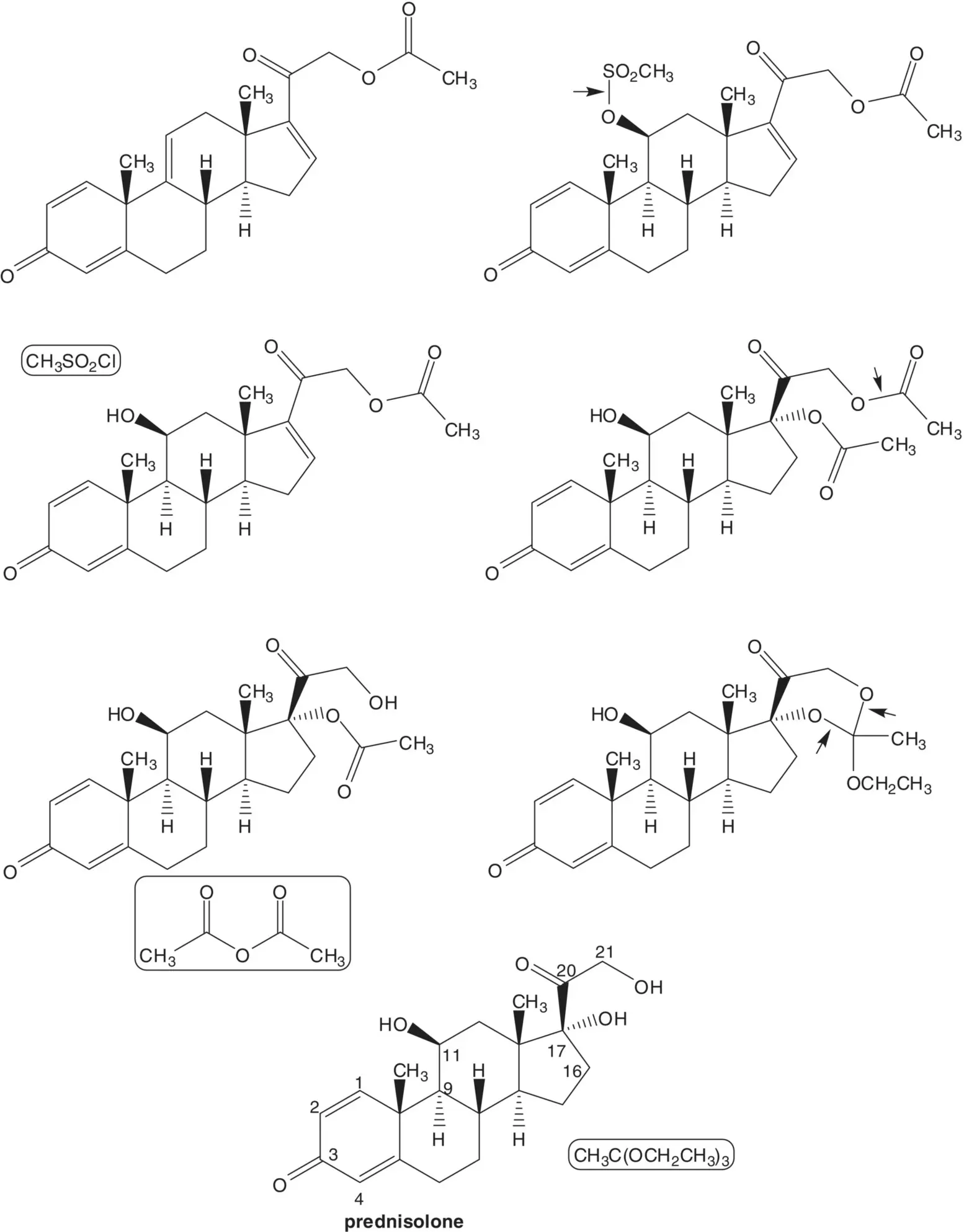
The 9(11)‐ene of the tetraene is formed by elimination of the C11β mesylate which is formed in situ. The 16‐alkene is formed by elimination of the 17α‐acetate of prednisolone 17α,21‐diacetate. Prednisolone 17α,21‐diacetate is formed by reaction of the 17α‐acetate with acetic anhydride. The 17α‐acetate is formed from prednisolone 17α,21‐ethyl orthoacetate. The orthoester is formed from prednisolone and triethyl orthoacetate.
Draw the structures of the retrosynthetic analysis of one alternative route to the key tetraene intermediate.
Anesthetics, Preoperative Medicines, and Medical Gases/Local Anesthetics
A piperidine is often formed by catalytic hydrogenation of a pyridine. Palladium, platinum, rhodium, ruthenium, and nickel catalysts have all been used. The hydrogenation is often run under acidic conditions to prevent catalyst deactivation by the piperidine product.
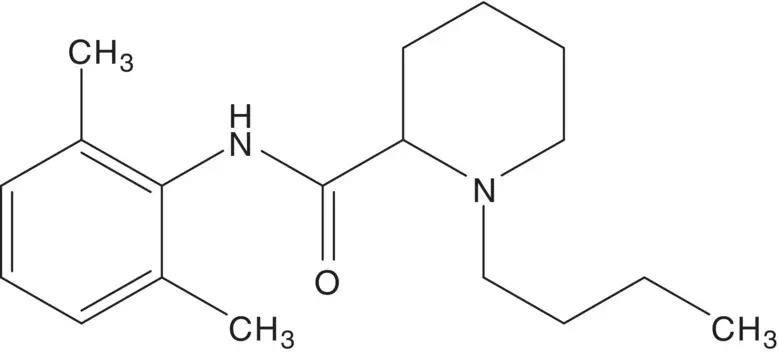
Discussion.Bupivacaine is a 1 : 1 mixture of enantiomers. Levobupivacaine, the ( S )‐enantiomer, is also manufactured for clinical use. The tertiary amine of bupivacaine is formed in the final step by N ‐alkylation of the secondary amine with 1‐bromobutane. The amide is formed from 2,6‐dimethylaniline (2,6‐xylidene) and the acid chloride. Pipecolic acid chloride hydrochloride is formed from pipecolic acid. Pipecolic acid is formed by catalytic hydrogenation of 2‐picolinic acid.
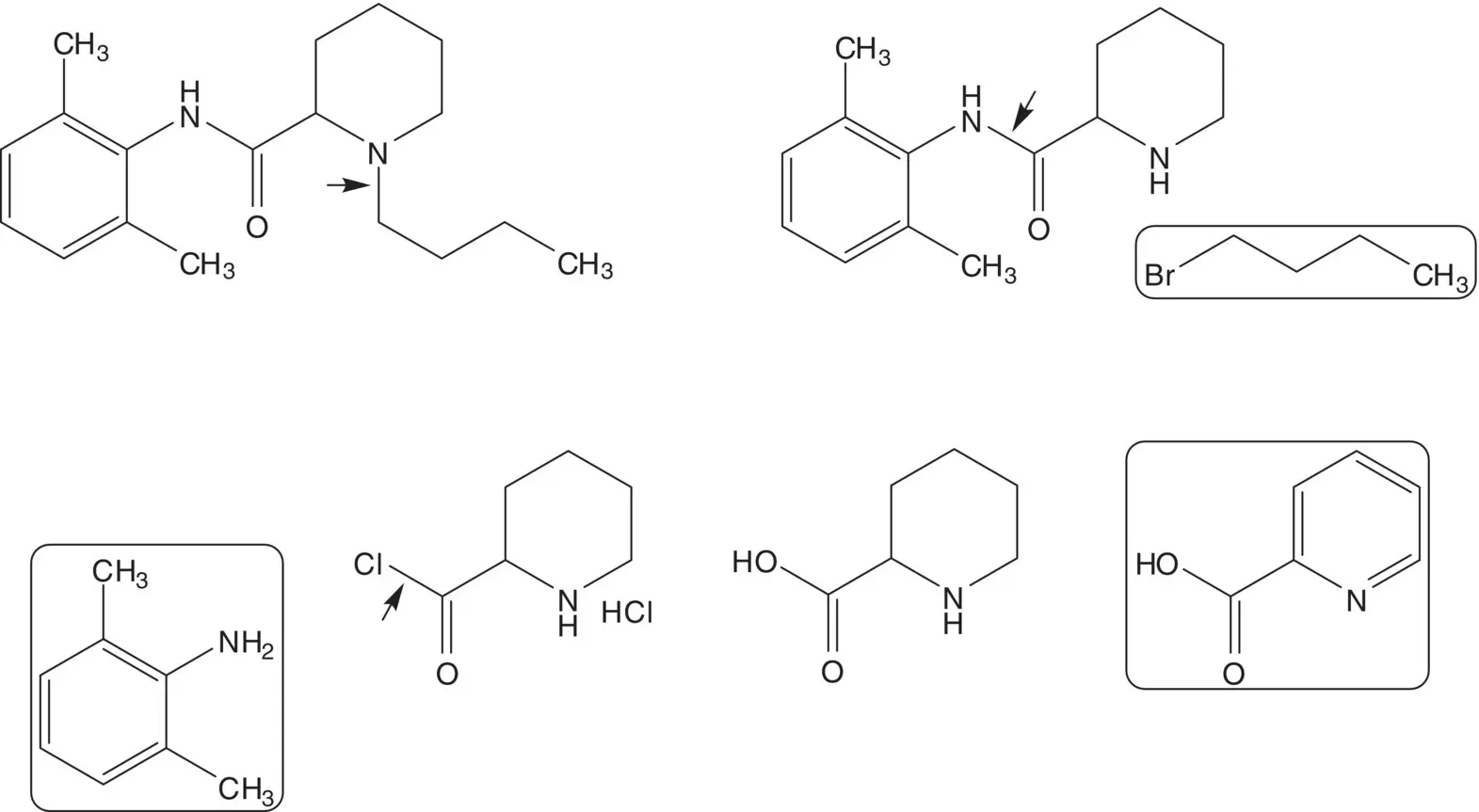
Draw the structures of the retrosynthetic analyses for three alternative routes to bupivacaine from the same starting materials (2,6‐dimethylaniline, picolinic acid, 1‐bromobutane) using the same transformations (catalytic hydrogenation, amide C─N bond formation, N ‐alkylation) in a different order.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «ЛитРес».
Прочитайте эту книгу целиком, купив полную легальную версию на ЛитРес.
Безопасно оплатить книгу можно банковской картой Visa, MasterCard, Maestro, со счета мобильного телефона, с платежного терминала, в салоне МТС или Связной, через PayPal, WebMoney, Яндекс.Деньги, QIWI Кошелек, бонусными картами или другим удобным Вам способом.





















