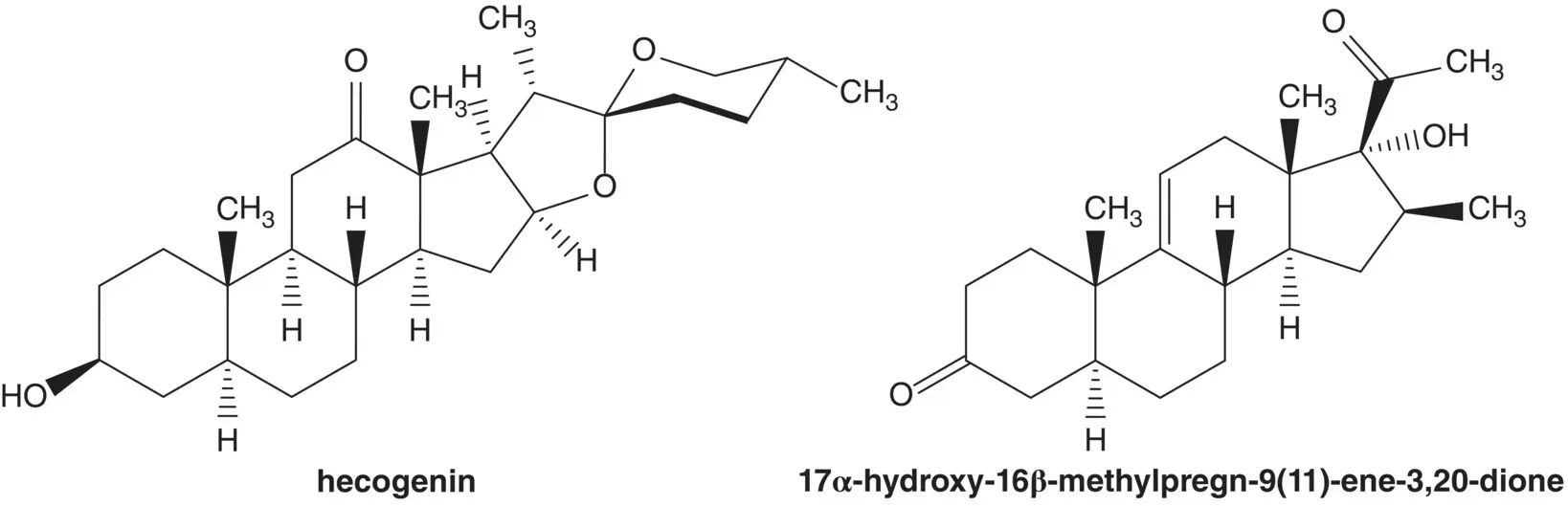
Discussion.Betamethasone is manufactured in 9 steps from 16β‐methylpregnane‐3β,17α,21‐triol 21‐acetate, 16 steps from 16‐dehydropregnenolone acetate, and 19 steps from diosgenin. Diosgenin is a phytosteroid sapogenin isolated from the tubers of Dioscorea wild yam.
Fluorine is introduced at C9 on the α–face in the final step by ring‐opening of the 9β,11‐epoxide with hydrogen fluoride. The 9β,11‐epoxide is formed from the bromohydrin. The 11β‐alcohol of the bromohydrin is formed in situ by hydrolysis of the formate ester. The 21‐alcohol is also released by hydrolysis of the carbonate ester. The bromohydrin formate is formed from the 9(11)‐alkene. The 9(11)‐alkene is formed by dehydration of the 11α‐alcohol. The C21 alcohol is protected as a carbonate.
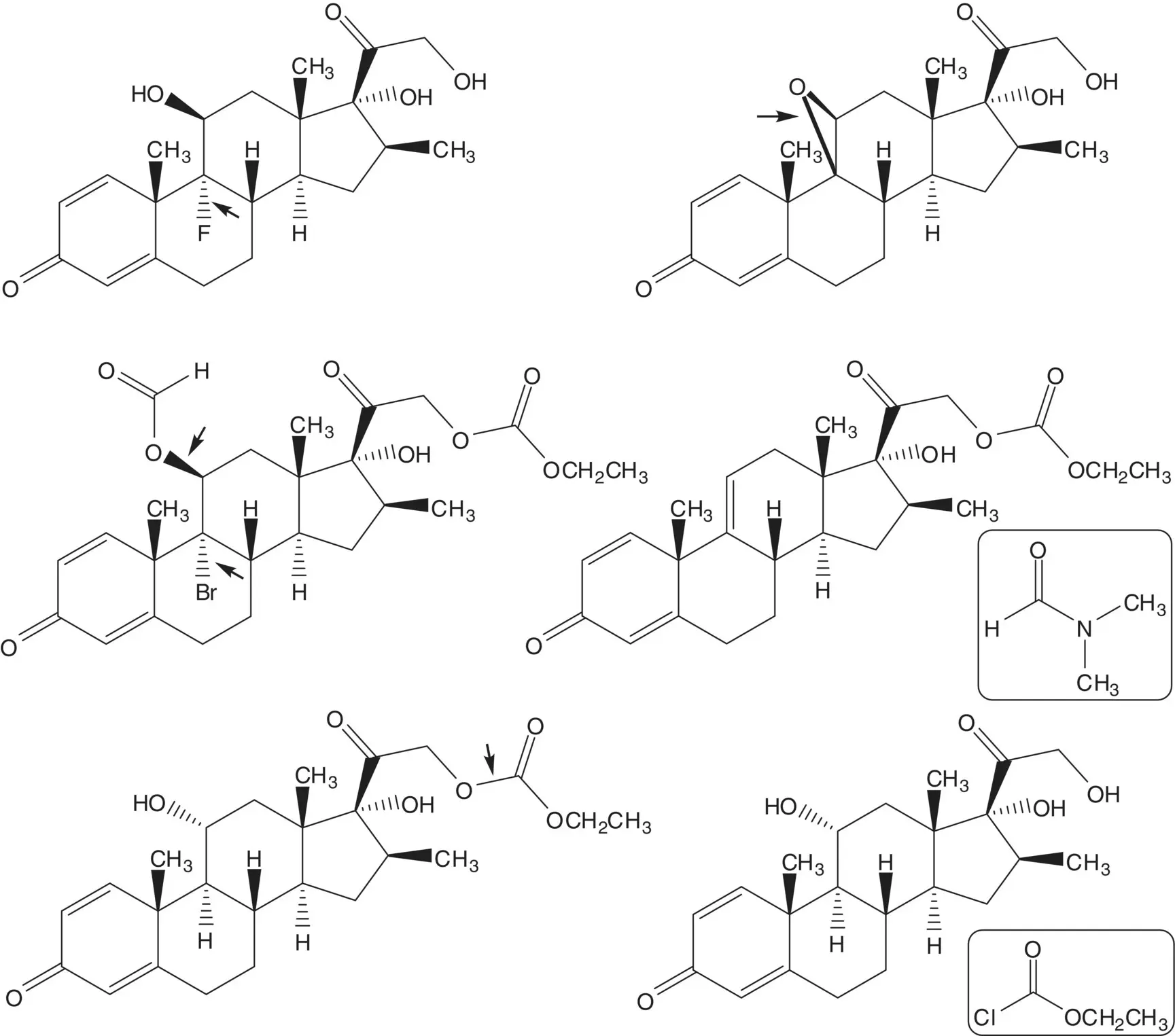
The 11α‐alcohol is formed by microbial oxidation. The acetate ester is hydrolyzed during the microbial oxidation. The 1,4‐diene‐3‐one is formed from the 2α,4α‐dibromo‐3‐one by double elimination. The 2α,4α‐dibromo‐3‐one is formed by α‐bromination of the C3 ketone. The C3 ketone is formed by oxidation of the C3 alcohol of 16β‐methylpregnane‐3β,17α,21‐triol 21‐acetate.
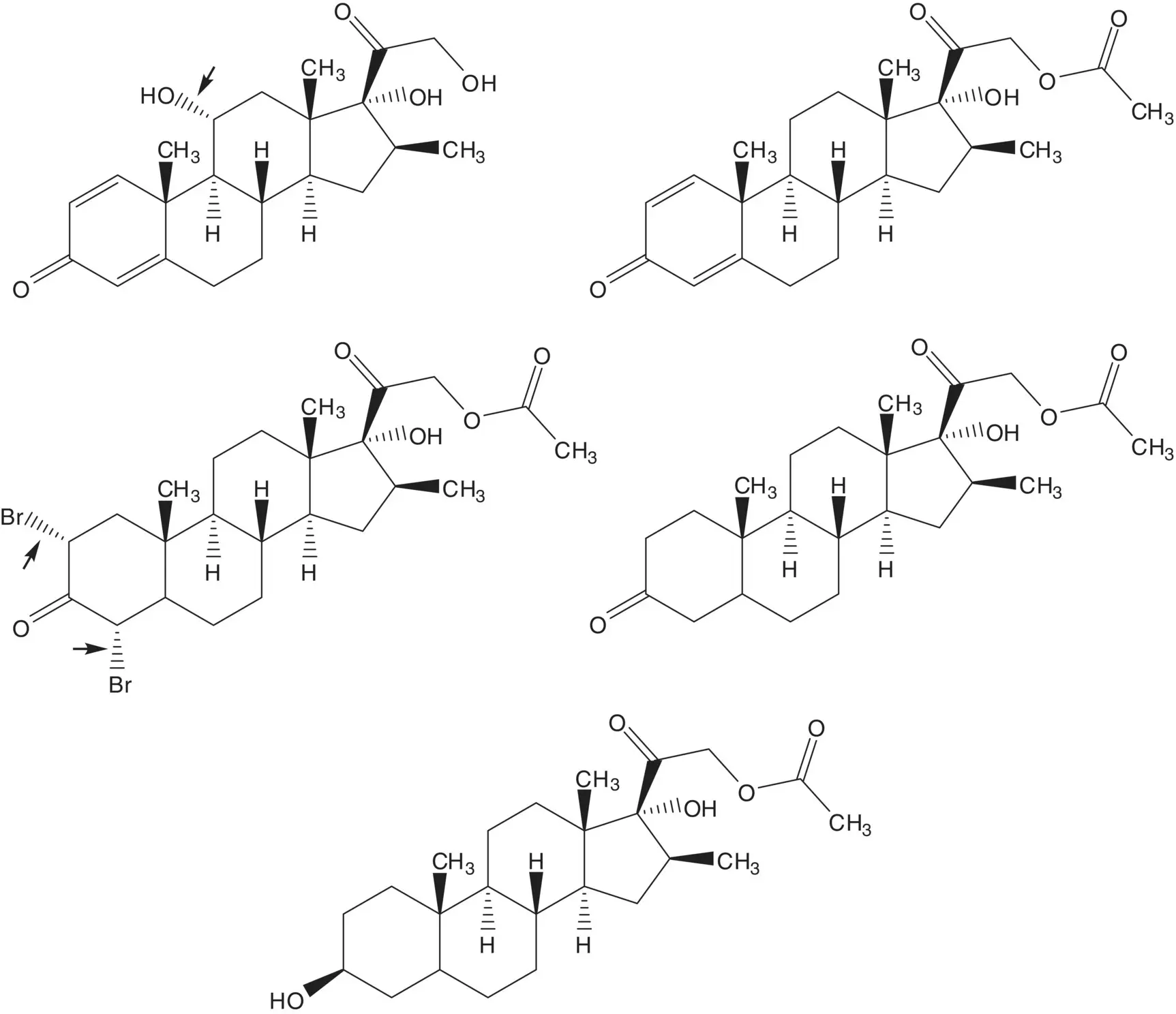
The acetate ester is formed by displacement of bromide by acetate. The α‐bromoketone is formed by bromination of the C20 ketone. The C20 ketone is released by acetal hydrolysis. The 16β‐methyl substituent is introduced by ring‐opening of the 16α,17‐epoxide with methylmagnesium bromide ( Grignard Reaction). Methylmagnesium bromide is formed from bromomethane. The 5,6‐alkene is reduced by catalytic hydrogenation. (What is the stereochemistry of the new chiral carbon at C5? Add this stereochemical feature to the structures in the retrosynthetic analysis.) The C20 ketone is protected as an acetal by reaction with ethylene glycol. The 16α,17‐epoxide is formed by epoxidation of the 16‐alkene of 16‐dehydropregnenolone acetate. The acetate ester is hydrolyzed under the epoxidation conditions.
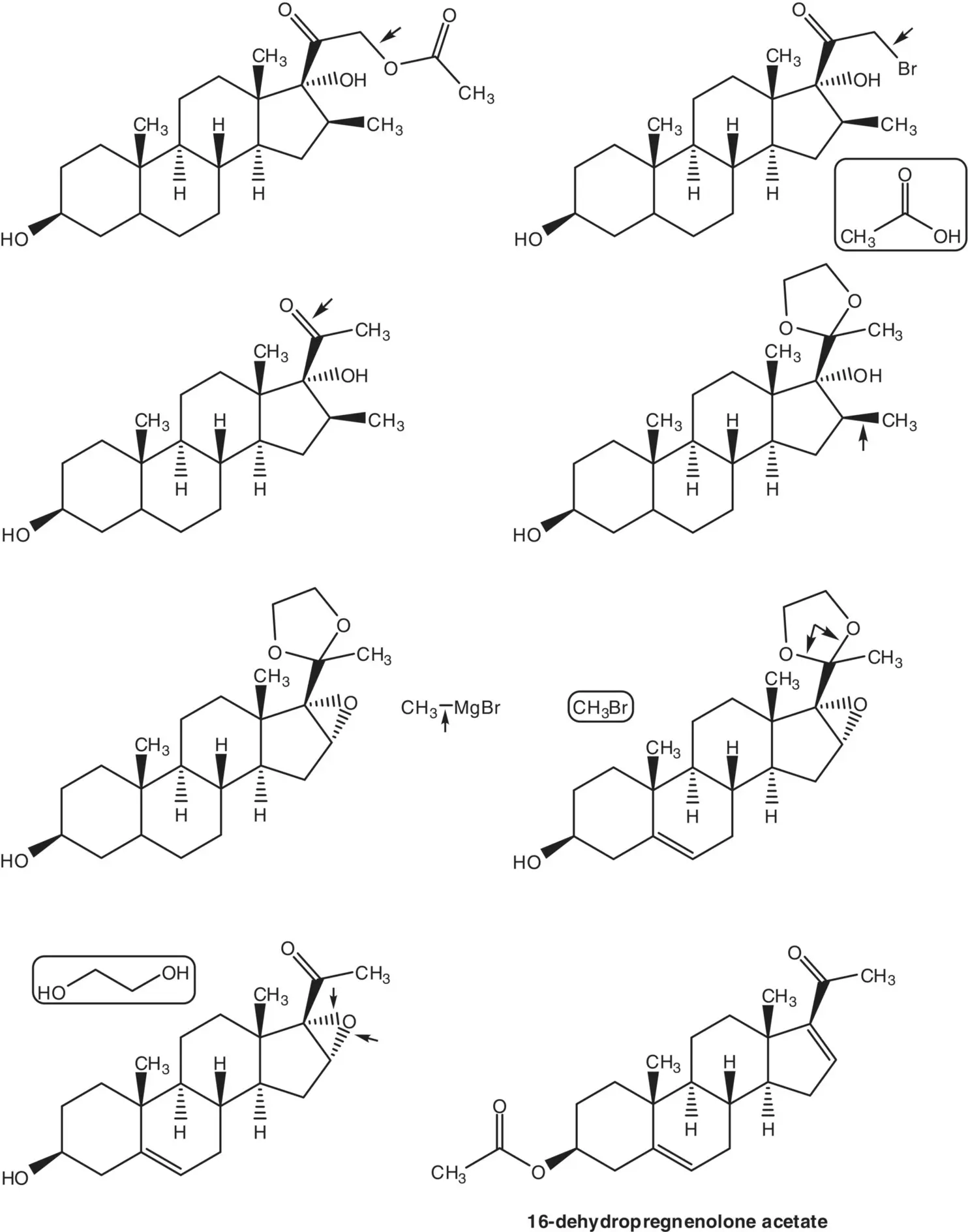
The 16‐alkene of 16‐dehydropregnenolone acetate is formed from diosone by β‐elimination. Diosone is formed by oxidation of the 20(22)‐alkene of pseudodiosgenin‐3,26‐diacetate. Pseudodiosgenin 3,26‐diacetate is formed by reaction of diosgenin with acetic anhydride. The three‐step synthesis of 16‐dehydropregnenolone acetate from diosgenin by acetylation, oxidation, and elimination is known as the Marker Degradation.
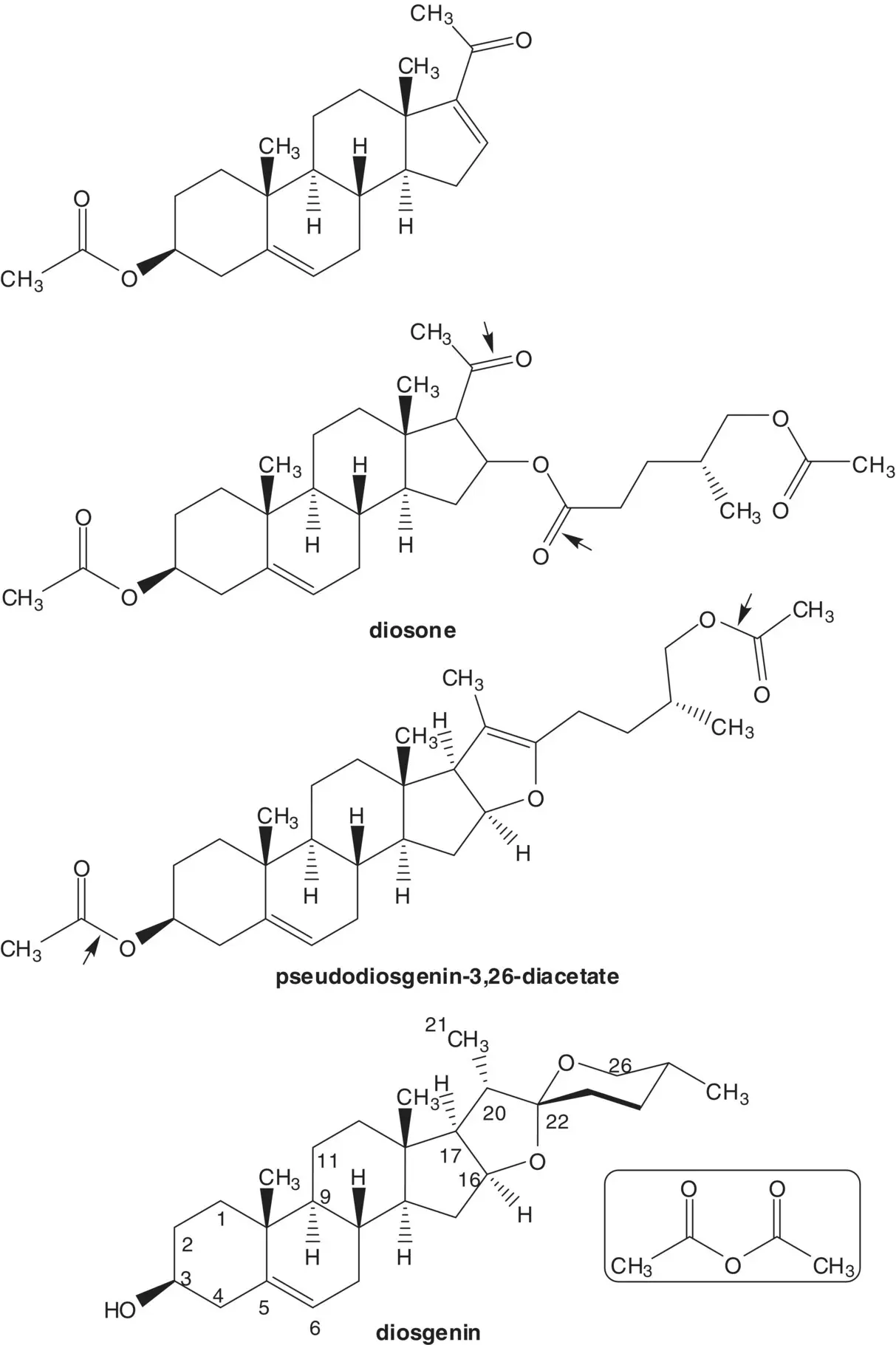
Hecogenin is a phytosteroid sapogenin isolated from the sisal plant, Agave sisalana . Draw the structures of the retrosynthetic analysis of an alternative route to betamethasone from hecogenin via 17α‐hydroxy‐16β‐methylpregn‐9(11)‐ene‐3,20‐dione.
Antineoplastics and Immunosuppressives/Hormones and Antihormones
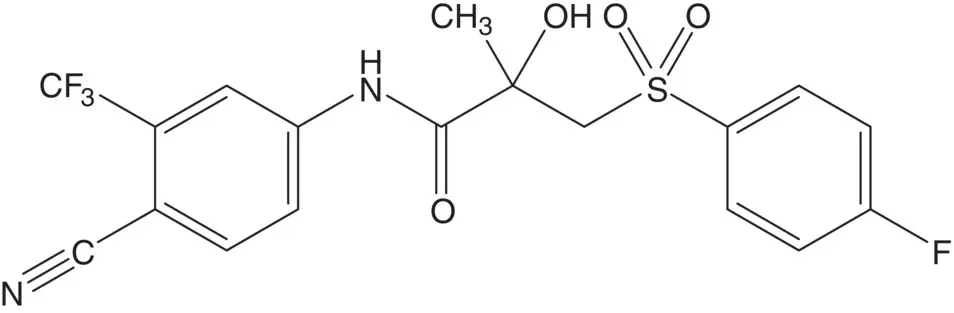
A β‐hydroxythioether is often formed by ring opening of an epoxide by a thiol.
Discussion.Bicalutamide is a 1 : 1 mixture of ( R )‐ and ( S )‐enantiomers. The sulfone is formed in the final step by oxidation of the thioether. The thioether is formed by ring opening of the epoxide by 4‐fluorothiophenol.
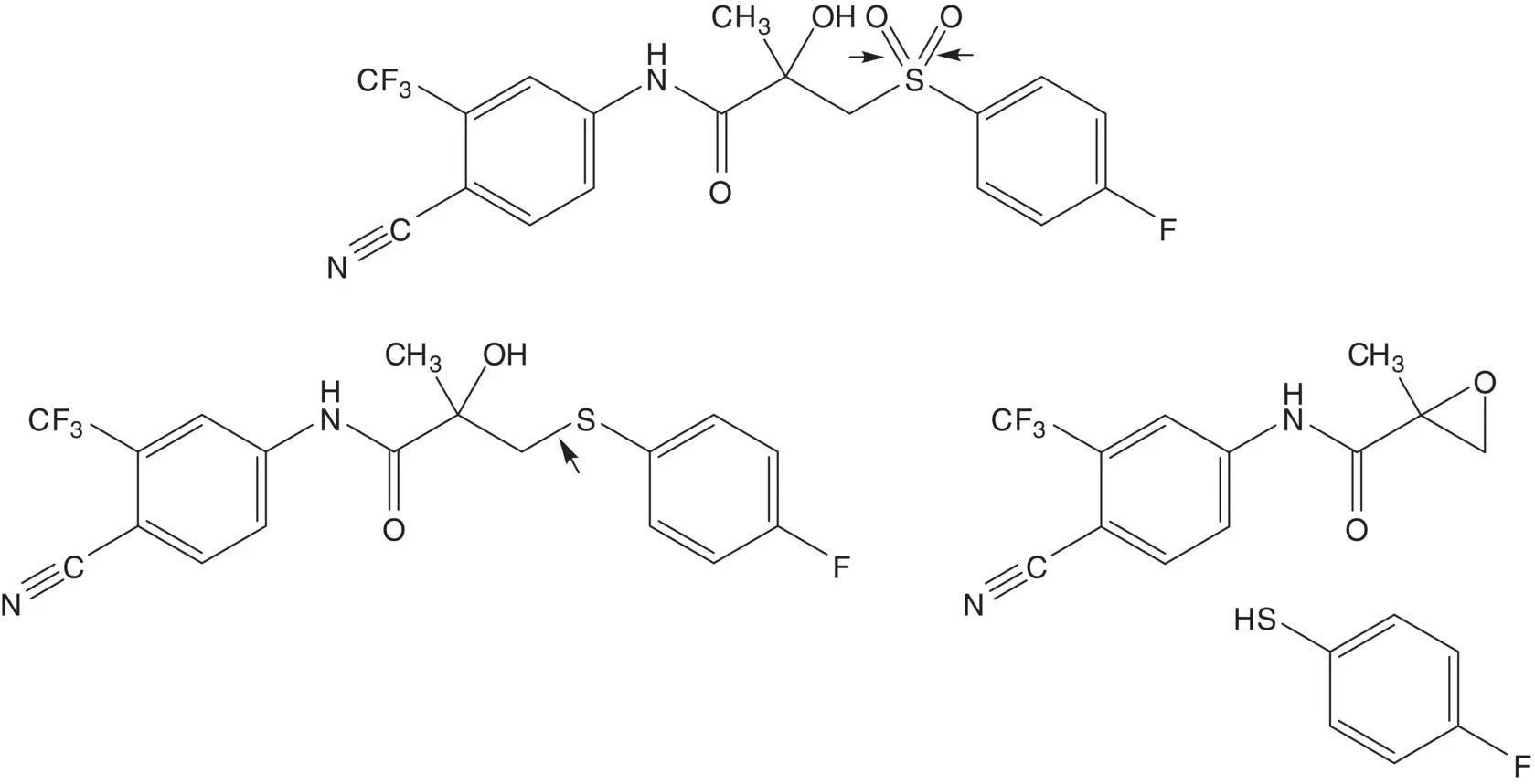
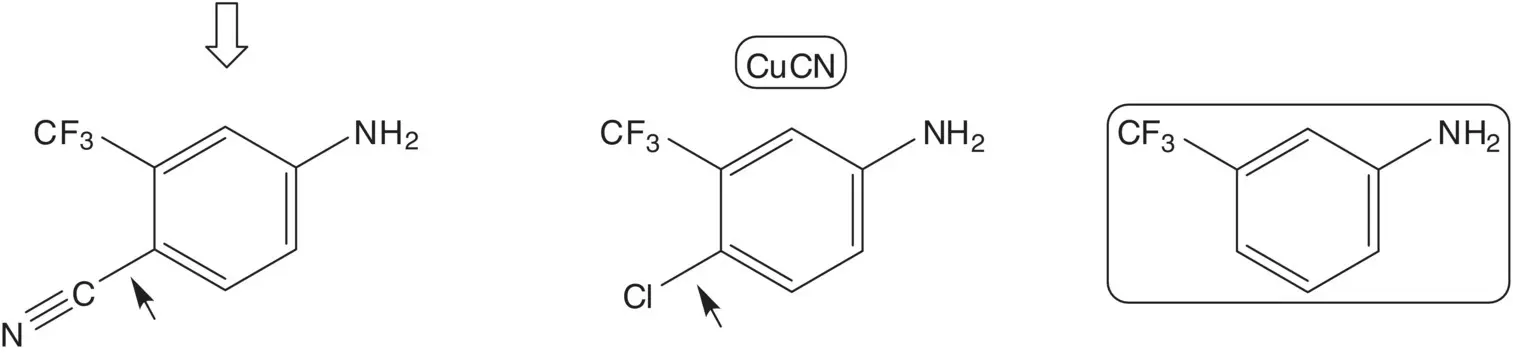
The epoxide is formed by epoxidation of the acrylamide. The acrylamide is formed by reaction of methacryloyl chloride with the amine. Methacryloyl chloride is formed from methacrylic acid. The amine, 4‐amino‐2‐(trifluoromethyl)benzonitrile, is formed by reaction of 4‐chloro‐3‐(trifluoromethyl)aniline with cuprous cyanide. 4‐Chloro‐3‐(trifluoromethyl)aniline is formed by chlorination of 3‐(trifluoromethyl)aniline. (How selective is the chlorination? What conditions are associated with the highest selectivity for formation of 4‐chloro‐3‐(trifluoromethyl)aniline?)
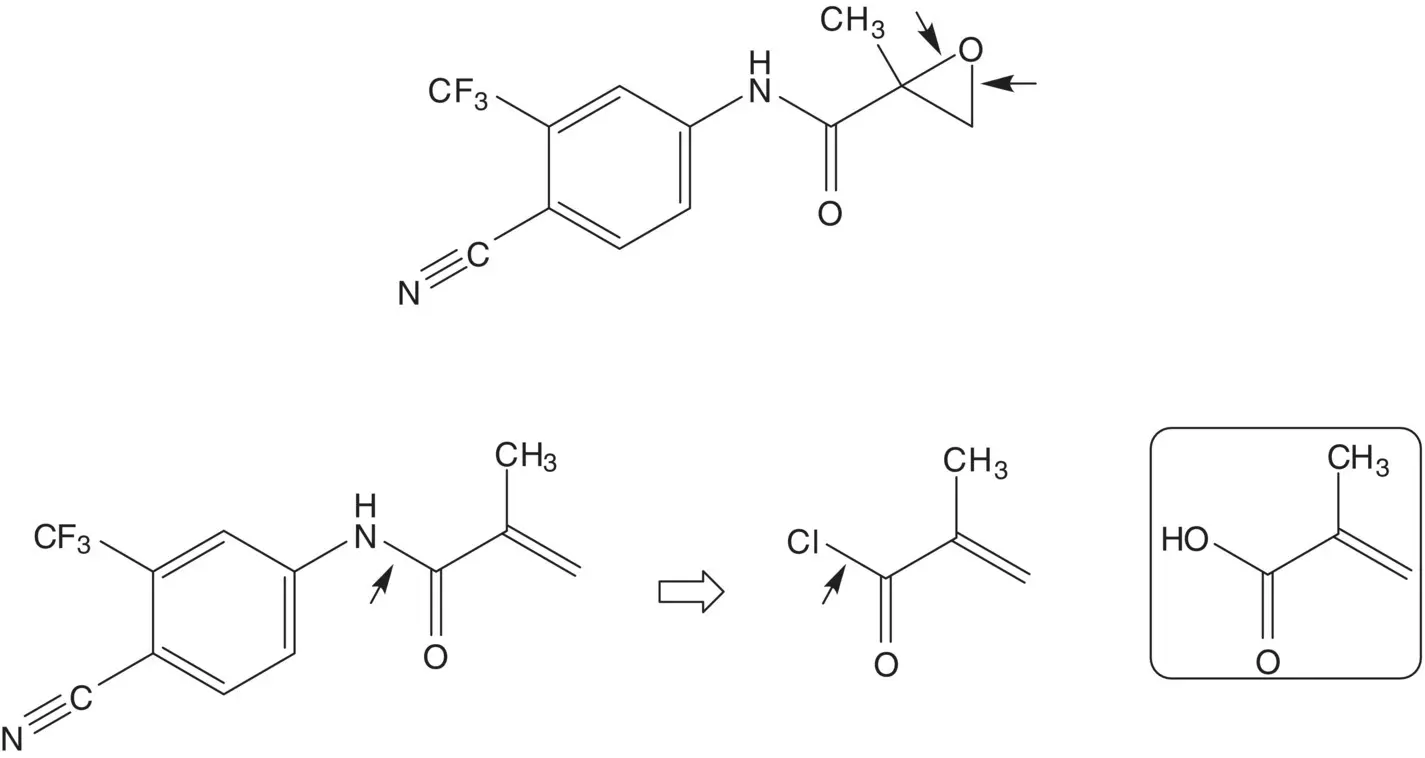
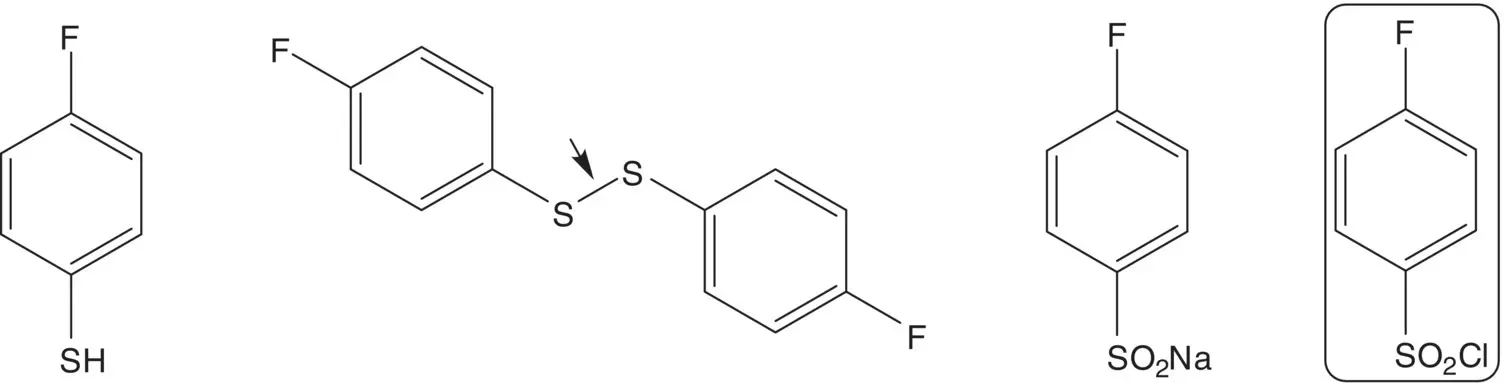
4‐Fluorothiophenol is formed by reduction of the disulfide. The disulfide is formed by reduction of the sulfinate salt. The sulfinate salt is formed by reduction of 4‐fluorobenzenesulfonyl chloride.
Draw the structures of the retrosynthetic analysis of one alternative route to bicalutamide which forms the C─S bond before forming the amide C─N bond. List pros and cons for both routes and select one route as the preferred route.
Antiparkinsonism Medicines
A tertiary alcohol is often formed by addition of an alkylmagnesium halide or arylmagnesium halide to a ketone (Grignard Reaction).
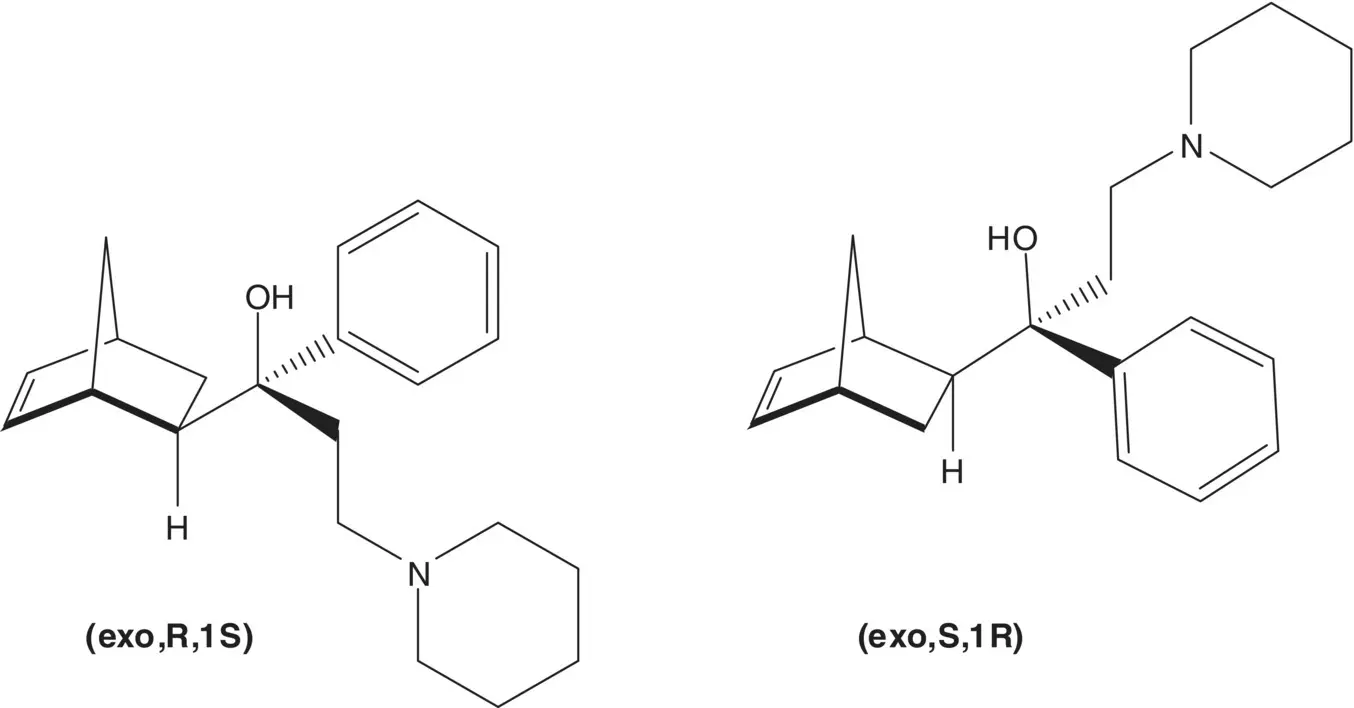
Discussion.Biperiden is a mixture of ( exo , R ,1 S )‐ and ( exo , S ,1 R )‐enantiomers. The retrosynthetic analysis of the ( exo , R ,1 S )‐enantiomer is shown. The tertiary alcohol is formed from phenylmagnesium chloride and the ketone ( Grignard Reaction). (How is biperiden separated from the mixture of stereoisomers that is formed in the Grignard Reaction?) Phenylmagnesium chloride is formed from chlorobenzene. The β‐piperidinoethyl ketone is formed by the reaction of the methyl ketone with formaldehyde and piperidine ( Mannich Reaction). (Some exo ‐to‐ endo isomerization is observed in the Mannich Reaction. What reaction conditions are associated with the minimum amount of exo ‐to‐ endo isomerization?) A mixture of four stereoisomers of 2‐acetyl‐5‐norbornene is formed in the [4 + 2]‐cycloaddition of cyclopentadiene with methyl vinyl ketone ( Diels–Alder Reaction). (Draw the structures of the stereoisomers.) The endo‐stereoisomers are isomerized to the exo‐stereoisomers.
Читать дальше























