1 ...6 7 8 10 11 12 ...21 Table 2.1 Selected examples of FDA‐ approved drugs containing spirocyclic motifs.
Source: Adapted from Knox et al. [2].
| Structure |
ID |
Name (Trade name) |
Indication |
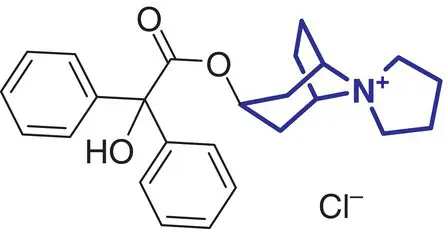 |
1 |
Trospium chloride (Flotros) |
Overactive bladder |
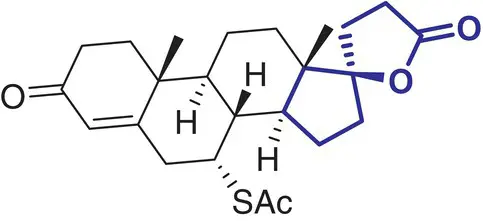 |
3 |
Spironolactone (Aldactone) |
Heart failure, edema, hypertension |
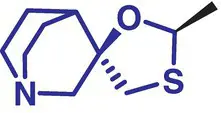 |
4 |
Cevimeline (Evoxac) |
Dry mouth (Sjögren's Syndrome) |
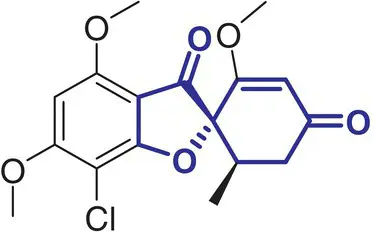 |
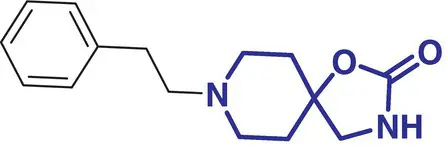
5 |
Griseofulvin (Crivicin) |
Antifungal antibiotic for ringworm infections |
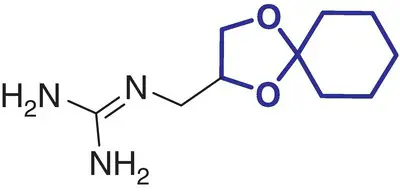 |
6 |
Guanadrel (Hylorel) |
Hypertension |
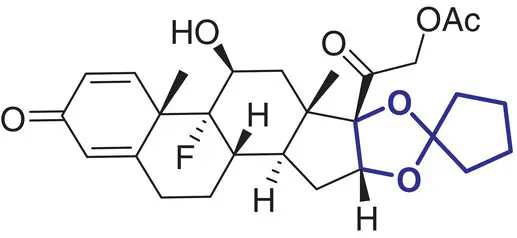 |
7 |
Amcinonide (Cyclocort) |
Inflammatory and pruritic manifestations |
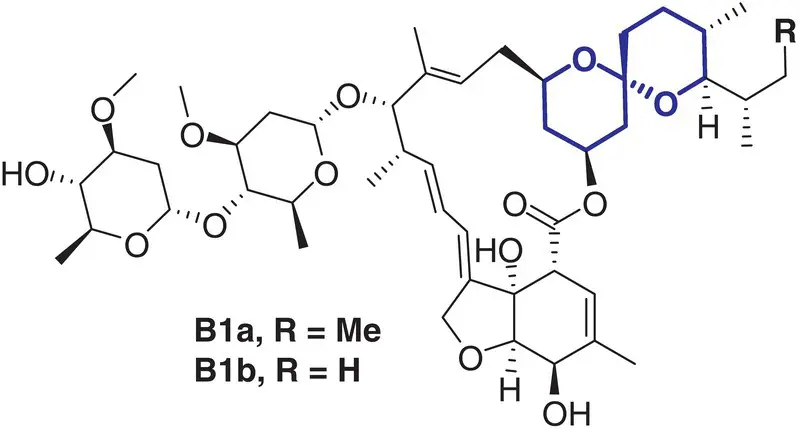 |
8 |
Ivermectin (Ascapil) |
Anti‐parasitic |
 |
9 |
Fenspiride (Eurespal) |
Antitussive |
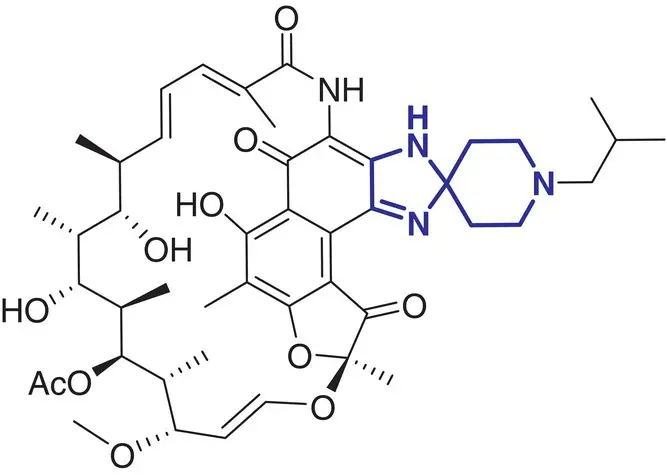 |
10 |
Rifabutin (Ansatipin) |
Antibiotic, tuberculosis |
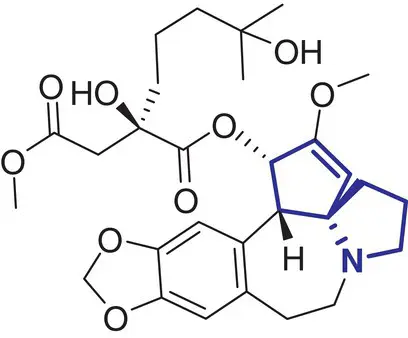 |
11 |
Homo‐harringtonine (Ceflatonin) |
Chronic myeloid leukemia |
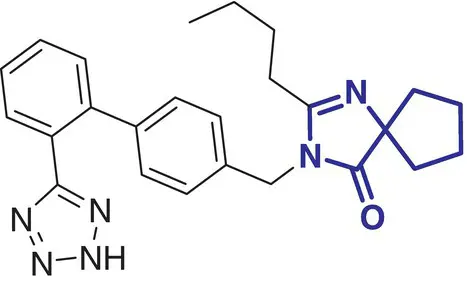 |
12 |
Irbesartan (Avapro) |
Hypertension |
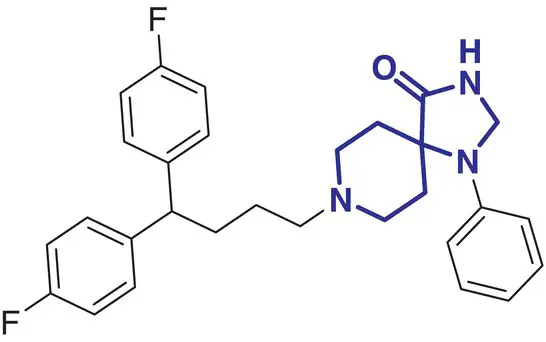 |
13 |
Fluspirilene (Imap) |
Schizophrenia |
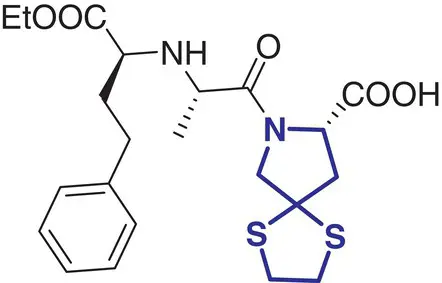 |
14 |
Spirapril (Renormax) |
Hypertension |
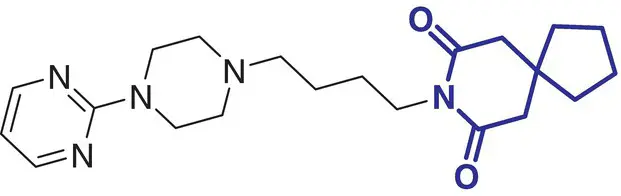 |
15 |
Buspirone (Buspar) |
Anxiety disorders |
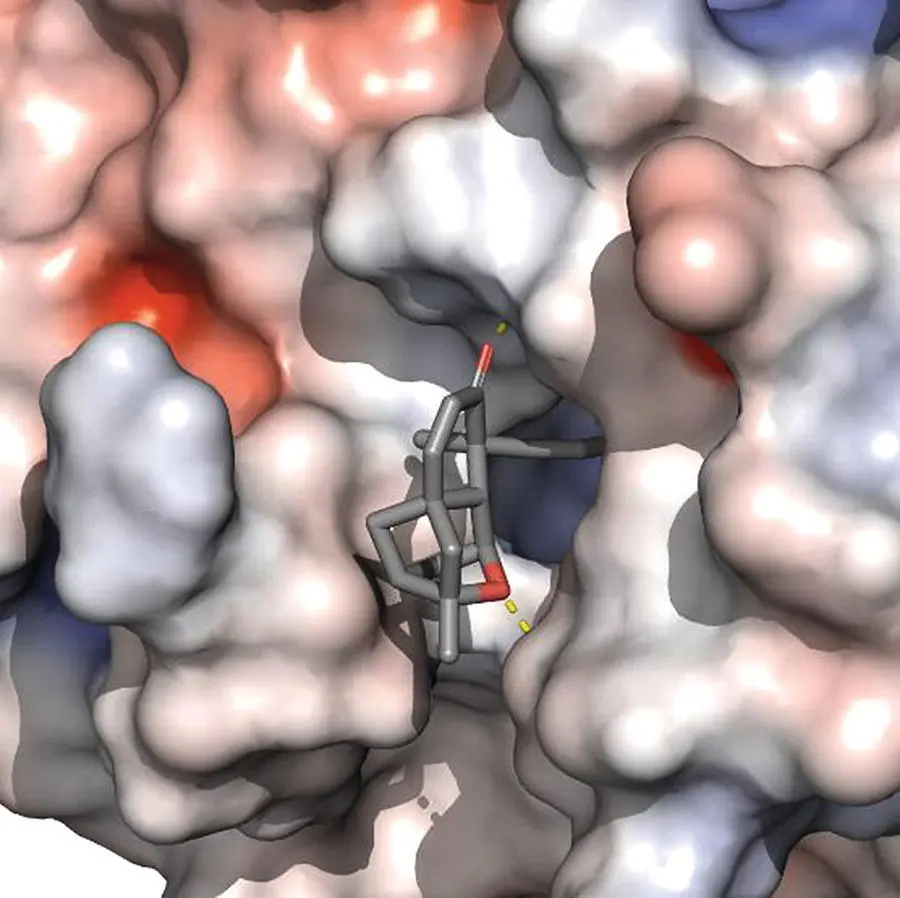
Figure 2.1 X‐ray crystal structure (pdb 2gfx 12) of Platensimycin ( 2, sticks representation) bound to the active site of FabF (surface representation) highlights hydrophobic contacts and hydrogen bonds in the complex.
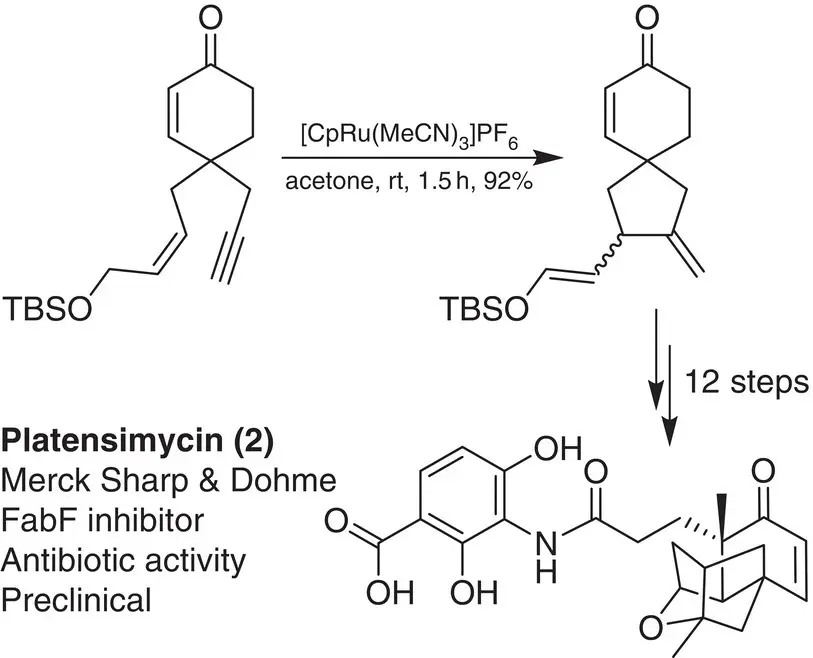
Scheme 2.1 Key enyne cycloisomerization step in Nicolaou’s total synthesis of platensimycin.
Source: Adapted from Nicolaou et al. [15].
In contrast, a comparatively small number of spirocycle containing drugs have been investigated in the last decades, and spirocyclic molecules are still under‐represented in marketed drugs [2]. Selected examples of spirocycle containing drugs are shown in Table 2.1, including spironolactone 3which has been known for over 50 years [20]. It seems fair to state that historically drug design strategies in medicinal chemistry have been heavily inspired (or biased?) by the advances in synthetic chemical methods toward new molecular scaffolds. This raises the question of chemical diversity and unconscious bias toward traditional/ubiquitous building blocks and reliable reactions/protocols for assembling increasingly complex molecules. As a compelling example, the phenyl ring and its related (hetero)aromatic analogues are over‐represented and virtually all best‐selling, over‐the‐counter drugs contain at least one such unit in their core scaffold or as a substituent. It is not particularly surprising as a plethora of synthetic methods for aromatic functionalization have been conceptually and experimentally well established for decades [21]. Such methods include, for example, a wide range of additions and substitutions, cross‐couplings, and aromatization strategies. While this is obviously a significant advantage for the rapid access to a wide range of analogues from a core structure, this bias is an important factor slowing down the discovery of new bioactive scaffolds. Historically, spirocycles have been considered difficult motifs to synthesize, mainly due to the presence of a quaternary carbon center. While chirality is often found in evolutionary optimized, complex natural product‐derived drugs (e.g. Griseofulvin 5,Ivermectin 8), early examples of synthetic spirocyclic drugs (e.g. fluspirilene 13) are achiral. The introduction of stereogenic centers allows higher structural complexity and diversity across the three dimensions, and it has been suggested to be advantageous for the discovery of ligands targeting biological receptors, such as an enzyme active site or a protein–protein interaction (PPI) interface [22]. Equally important is the control of their configuration for achieving optimal interaction with the chiral target receptor. It is well illustrated by the contrasted potencies and/or pharmacologies of various stereoisomers of a wide range of chiral drugs [23, 24], including the infamous thalidomide [25]. Controlling the stereochemical outcome, hence chirality of spirocycles, has been a major focus of synthetic organic chemists in the last decade, and this is reflected by the exponentially growing number of articles in organic chemistry journals reporting the syntheses of increasingly complex spirocyclic systems. Overall, spirocycles have gained increasing popularity in modern drug discovery, and the recent development of robust synthetic methodologies have made them an integral part of the medicinal chemists’ arsenal of pharmacophores for the development of small synthetic molecules targeting therapeutically important biological pathways. This brief chapter captures several important literature examples that illustrate some key applications of spirocycles for the development of bioactive molecules of relevance to the pharmaceutical sector. The breadth of synthetic methods available for the preparation of a wide range of spirocycles will be discussed in the subsequent chapters.
Читать дальше