Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
We wish once more to draw the attention of the readers on the potential usefulness and uniqueness of the spiro motif in the interaction with a specific biological target spanning from drugs to agrochemicals.
The enzyme Acetyl‐coenzyme A carboxylases (ACCs) have crucial roles in fatty acid metabolism in most living organisms, among which include humans, insects, and plants. The experimental ACC inhibitor compounds for the treatment of human metabolic disease contain a spirocyclic moiety as in Takeda compound 19[15] and in Pfizer PF‐05221304 20. The last one is currently in phase II clinical trials for the treatment of Non‐Alcoholic Steatohepatitis (NASH) [16] ( Figure 1.7).
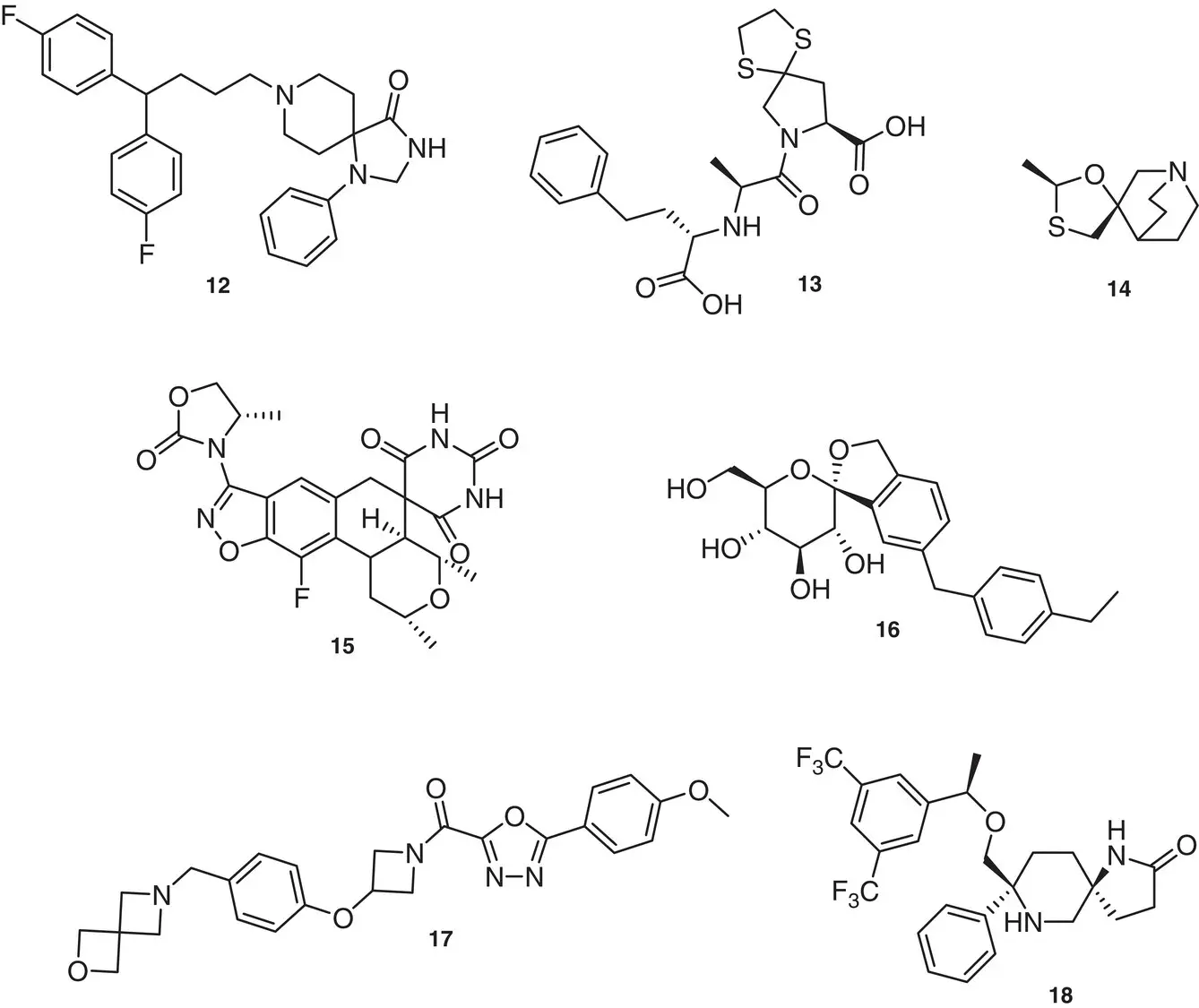
Figure 1.6 Examples of marketed spiro compound drugs.
Sources: Based on Zheng and Tice [13]; Zheng et al. [14].
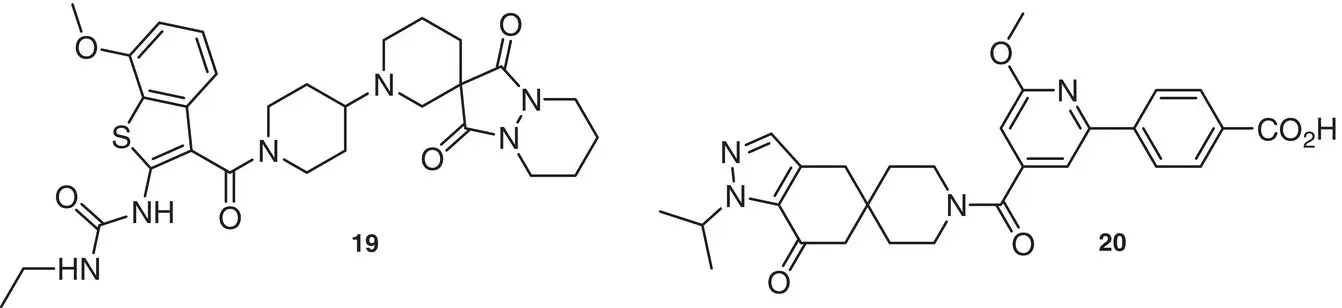
Figure 1.7 ACC inhibitors of pharmaceutical interest.
Sources: Based on Bourbeau and Bartberger [16a]; Esler and Bence [16b].
The commercial insecticide/acaricide products spirotetramat 21, spiromesifen 22, and spirodiclofen 23from Bayer CS and spiropidion 24from Syngenta, acting as insect ACC inhibitors, all have spirocyclic structures [17] ( Figure 1.8).
New spirocyclic herbicide compounds with the representative formula 25have been recently patented [18]. It is noteworthy that compounds 21and 24, sharing similar molecular features with 25, do not show any phytotoxic effect ( Figure 1.9).
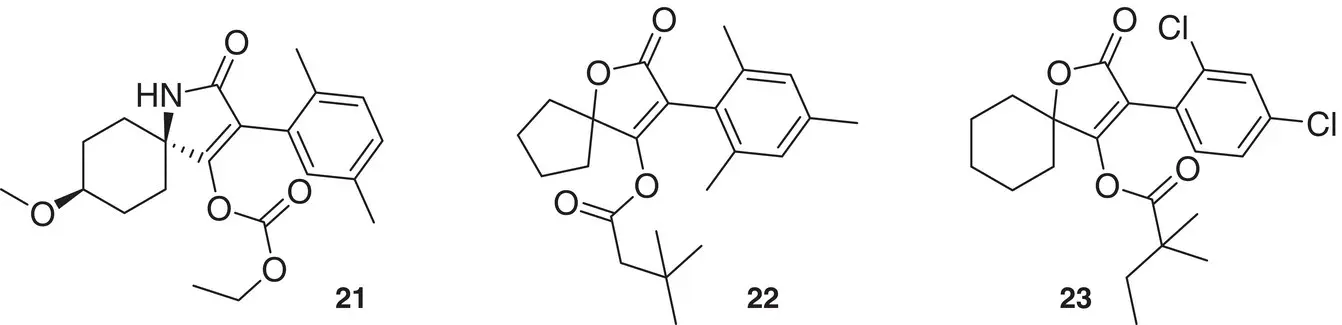
Figure 1.8 Commercial spirocyclic insecticide/acaricide products.
Source: Jeschke et al. [17].
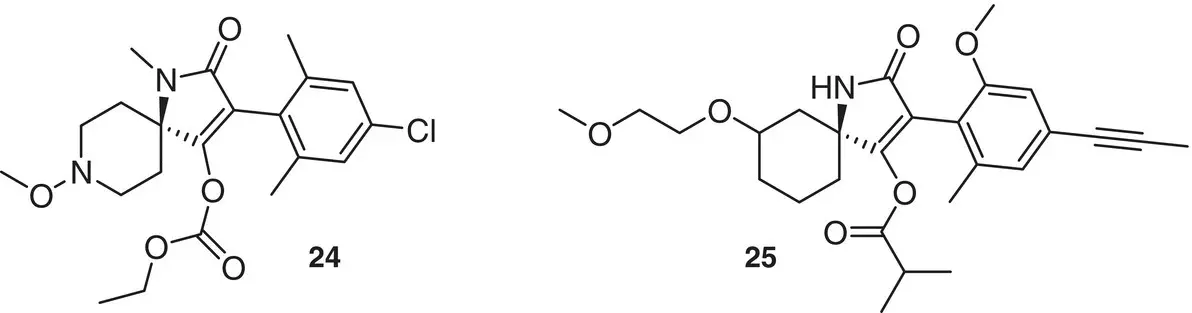
Figure 1.9 Recently patented spiro compound of agrochemical interest.
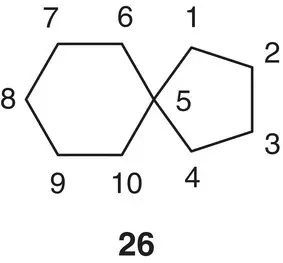
Figure 1.10 Example of numbering of spirocyclic compounds.
As presented in this chapter, spirocyclic scaffolds find application in a large number of sectors for their own peculiar architecture characteristics, displaying valuable application properties, or simply because of the introduction of structural novelty that guarantee patentability and intellectual property rights.
1.1 Notes on IUPAC Rules for Spiro Compounds
Naming spirocycles could be quite complex. The accepted rules are collected in the IUPAC blue book [1, 19].
Simplifying with two examples, the structure 26is numbered starting from the smallest cycle ( Figure 1.10). The name comes from the prefix spiro followed by square brackets containing the number of atoms of the two cycles starting from the smallest and excluding the spirocenter. In this case, the functional group is an alkane so that the name became spiro[4,5]decane.
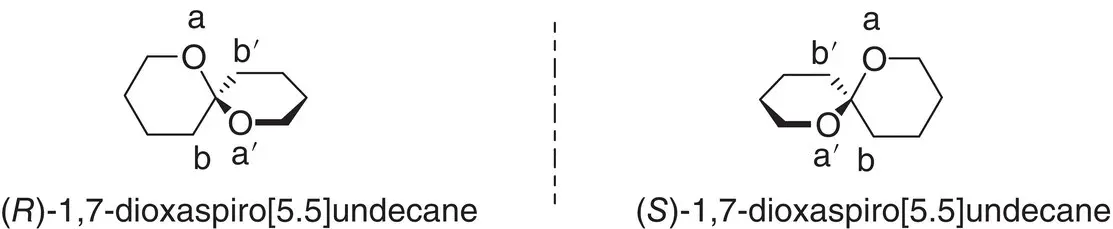
Figure 1.11 Example of naming chiral spiro compounds.
When the compound is chiral because it contains a chiral center, the CIP rules are followed. In the case in which the substituents on the spirocenter are the same, but the structures display an axial chirality as in Figure 1.11, we assign arbitrarily the priority to one of the cycles and then, within each cycle the order follows the CIP rules: a>a′>b>b′.
References
1 1 Moss, G. (1999). Pure Appl. Chem. 71: 531–558.
2 2 Baeyer, A.V. (1900). Ber. Dtsch. Chem. Ges. 33: 3771–3775.
3 3 (a) Singh, G.S. and Desta, Z.Y. (2012). Chem. Rev. 112: 6104–6155. (b) Smith, L.K. and Baxendale, I.R. (2015). Org. Biomol. Chem. 13: 9907–9933.
4 4 Fleming, I. (2010). Molecular Orbitals and Organic Chemical Reactions, Reference Edition. Chichester, UK: Wiley & Sons, Ltd.
5 5 Molvi, K.I., Haque, N., Awen, B.Z.S., and Zameeruddin, M. (2014). World J. Pharm. Sci. 3: 536–563.
6 6 Saragi, T.P.I., Spehr, T., Siebert, A. et al. (2007). Chem. Rev. 107: 1011–1065.
7 7 Lupo, D., Salbeck, J., Schenk, H. et al. (1998). Spiro compounds and their use as electroluminescence materials. Patent: US5840217, 24 November 1998.
8 8 Wössner, J.S., Grenz, D.C., Kratzert, D., and Esser, B. (2019). Org. Chem. Front. 6: 3649–3656.
9 9 Xu, J.‐P. (2017). Cancer Inhibitors from Chinese Natural Medicines. Boca Raton, FL: CRC Press.
10 10 Cruz, M.S., Barroso, S.C., Navoni, J.A. et al. (2016). Toxicol. Rep. 3: 539–543.
11 11 Jossang, A., Jossang, P., Hadi, H.A. et al. (1991). J. Org. Chem. 56: 6527–6530.
12 12 Hart, D.J. (2011). Organic Synthesis via Examination of Selected Natural Products. Singapore: World Scientific Publishing Co Pte Ltd.
13 13 Zheng, Y. and Tice, C.M. (2016). Expert Opin. Drug Discovery 11: 831–834.
14 14 Zheng, Y., Tice, C.M., and Singh, S.B. (2014). Bioorg. Med.Chem. Lett. 24: 3673–3682.
15 15 Kamata, M., Yamashit, T., Kina, A. et al. (2012). Bioorg. Med. Chem. Lett. 22: 4769–4772.
16 16 (a) Bourbeau, M.P. and Bartberger, M.D. (2015). J. Med. Chem. 58: 525–536. (b) Esler, W.P. and Bence, K.K. (2019). Cell. Mol. Gastroenterol. Hepatol. 8: 247–267.
17 17 Jeschke, P., Witschel, M., Krämer, W., and Schirmer, U. (eds.) (2019). Modern Crop Protection Compounds, 3e. Weinheim, Germany: Wiley‐VCH Verlag GmbH.
18 18 Angermann, A., Bojack, G., Buscato Arsequell, E., et al. (2019). Specifically substituted 2‐alkyl‐6‐alkoxyphenyl‐3‐pyrrolin‐2‐ones and their use as herbicides. Patent: WO2019219588, 13 May 2019.
19 19 Favre, H.A. and Powell, W.H. (2013). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names. RSC.
2 Selected Applications of Spirocycles in Medicinal Chemistry
Matthias Baud
School of Chemistry and Institute for Life Sciences, University of Southampton, Southampton, UK
2.1 Introduction
Spiro compounds contain two rings, connected by a single sp 3hybridized quaternary center, the “spiroatom” [1]. The latter is often a carbon, although a number of quaternary N ‐spiro ammoniums have also been reported. Trospium chloride ( 1) ( Table 2.1) is a good example, and its spiro ammonium motif can be readily prepared by double N ‐alkylation of endo‐nortropine [3]. Spirocyclic systems are found in a wide range of natural products [4], including spiro‐ketals [5, 6], lactones [7], lactams [8, 9], and oxindoles [10–12]. An early and illustrative example of spirocyclic natural product which has attracted the attention of medicinal chemists is the antibiotic platensimycin ( 2). It is a metabolite from Streptomyces platensis which represents a structurally unusual example of bioactive molecule containing a carbaspirocyclic scaffold. Its antibiotic activity was reported by Merck in 2006, as part of a screening campaign to identify inhibitors of beta‐ketoacyl synthases I/II (FabF/B) enzymes [13]. Inhibition of FAB enzymes by platensimycin leads to impaired biosynthesis of key fatty acids required bacterial cell membrane integrity [14]. Platensimycin displays activity against a range of Gram‐positive bacteria, including strains showing resistance to other potent antibiotics such as methicillin, vancomycin, linezolid, or macrolide. Structural studies on an Escherichia coli FabF(C163Q) in complex with platensimycin highlighted important interactions underlying complex formation. The shape complementarity and conformational restriction provided by the spiro motif are important contributors to the potency of platensimycin, allowing polar interactions and hydrophobic contacts at the binding site entrance ( Figure 2.1) [13]. The first total synthesis of racemic platensimycin was reported by Nicolaou on the same year ( Scheme 2.1) [15], involving a key ruthenium‐catalyzed enyne cycloisomerization [16]. Since then, stereoselective syntheses of platensimycin spirocyclic core based on rhodium‐catalyzed asymmetric cycloisomerization and hypervalent iodine‐mediated de‐aromatizing cyclization [17], decarboxylative allylation [18], and intramolecular Diels–Alder [19] have been reported.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.