Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
I also want to dedicate this book to Professor Dieter Enders who left us during the writing of this book. He was always a source of inspiration, since his early success with RAMP and SAMP chemistry, until the development of highly complex domino reactions, showing a commitment and brilliance to organic chemistry that inspired me in my career. I still remember his kind hospitality in The Domino cat symposium in Aachen. Professor Enders, you left a huge footprint in organic and synthetic chemistry.
Finally, I want to thanks all the authors for their work and commitment in those difficult COVID times.
1 Spiro Compounds: A Brief History
Marta Meazza
School of Chemistry, University of Southampton, Southampton, UK
Policyclic molecules containing at least two rings joined together by a single atom, mostly a carbon atom, previously named spiranes, are called spiro compounds or spirocycles, and the single central atom is referred to as the spiro atom [1]. We should mention that apart from carbon, other elements such as nitrogen, phosphorus, and arsenic may represent the spiro atom.
The term was coined by the German chemist Nobel laureate Adolf von Baeyer who created the first spirane in 1900 [2].
This peculiar structural feature is present in natural products and has long been the subject of methodological studies and synthetic efforts [3].
Several synthetic procedures for spiro compounds have been developed and will be extensively discussed in the next chapters. However, the asymmetric synthesis of spirocycles that allow the creation of stereogenic quaternary centers represent a demanding task for organic chemists. Even the concepts of spiro aromaticity and spiro antiaromaticity can be applied when spiroconjugation is possible [4].
The search for the key term “spiro” in SciFinder ndatabase, at the end of October 2019, resulted in more than 40 700 references with an exponential growth starting from the middle of the last century and an increasing attention to this subject is expected in the future ( Figure 1.1).
These massive research efforts cover a wide range of fields from organic and medicinal chemistry to material sciences and engineering, to name a few.
The enormous interest in spiro compounds rely on their distinctive properties often associated with the three‐dimensional stereochemical features, reflecting on their pharmacological properties that include, among others, bactericidal, fungicidal, anticancer, cytotoxic, antidepressant, antihypertensive, insecticidal, herbicidal, and plant growth regulatory effects [5]. These properties are due to the tetrahedral nature of the spiro carbon and consequent asymmetric features associated with it.
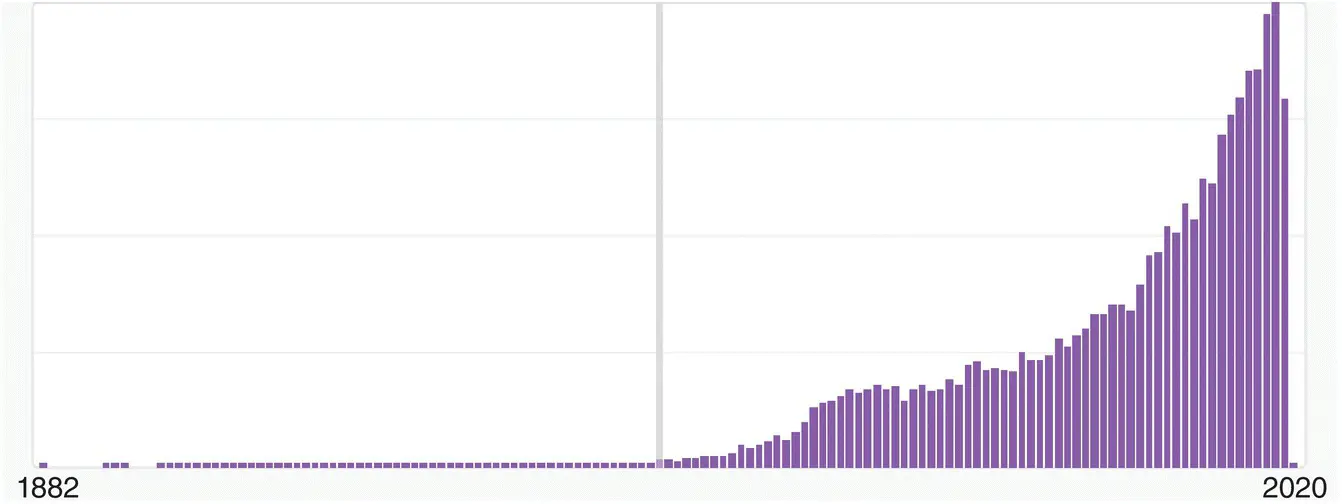
Figure 1.1 Growing interest in spiro compounds in chemical literature.
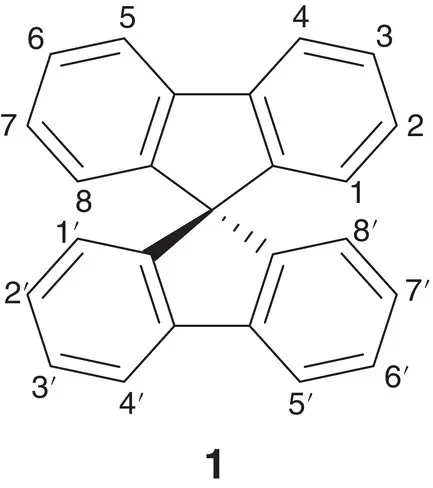
Figure 1.2 Dye sensitizer 9,9‐spirobifluorene.
Source: Lupo et al. [7].
In addition, many other practical utilizations include optoelectronic devices, ophthalmic lenses, and solar cells [6]. Compounds like 9,9‐spirobifluorene 1( Figure 1.2) have application in dye‐sensitized solar cells (DSCs) and represent the most efficient alternative to the current solar cell technologies [7].
Spirocyclic compounds find technological application as efficient charge‐transfer molecules due to their intramolecular donor–acceptor structural feature amplified by spiroconjugation. The desired optical properties can be achieved by careful design of the spiro donor–acceptor characteristic as illustrated in Figure 1.3[8]. When structural characteristics make it possible, spiro compounds can equilibrate with their non‐spiro analogues exhibiting photochemical phenomena like photochemical memory.
We report here some examples of carbocyclic and heterocyclic naturally occurring compounds containing the spiro moiety ( Figure 1.4). One of the simplest compounds is the pheromone of the olive fly Dacus oleae 5. Phelligridin G 6from the fungus Phellinus igniarius has been long used in Traditional Chinese Medicine for the treatment of gonorrhea [9]. The antimycotic drug griseofulvin 7, isolated from a penicillium mold in 1939, found application in the treatment of fungal skin infections since 1957. Hecogenin 8, the aglycone part of a steroid saponin found in the plant Agave sisalana, is responsible for many therapeutic effects and is also used as a starting material in the synthesis of corticosteroids [10]. Horsfiline 9is an oxindole alkaloid having analgesic effect, isolated from the plant Horsfieldia superba [11].
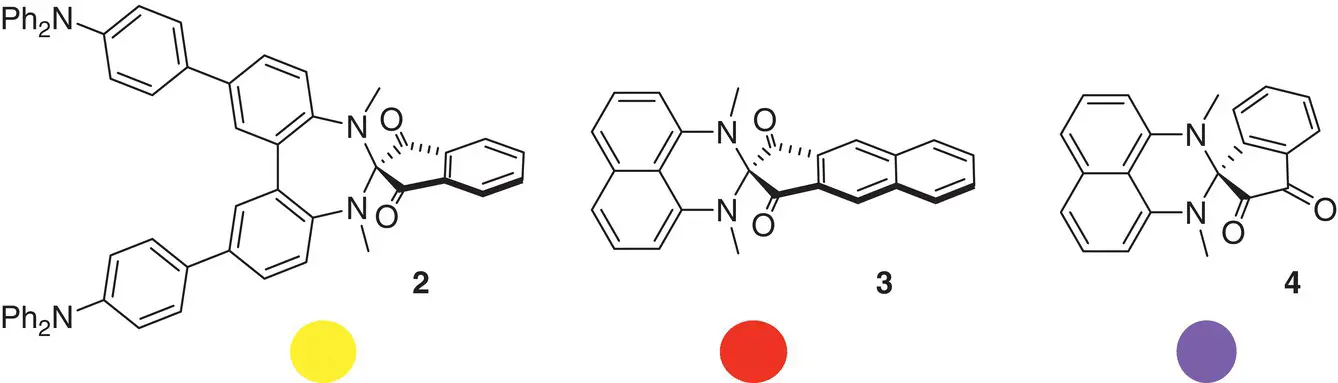
Figure 1.3 Donor–acceptor spiro compounds and colors displayed by them.
Source: Wössner et al. [8].
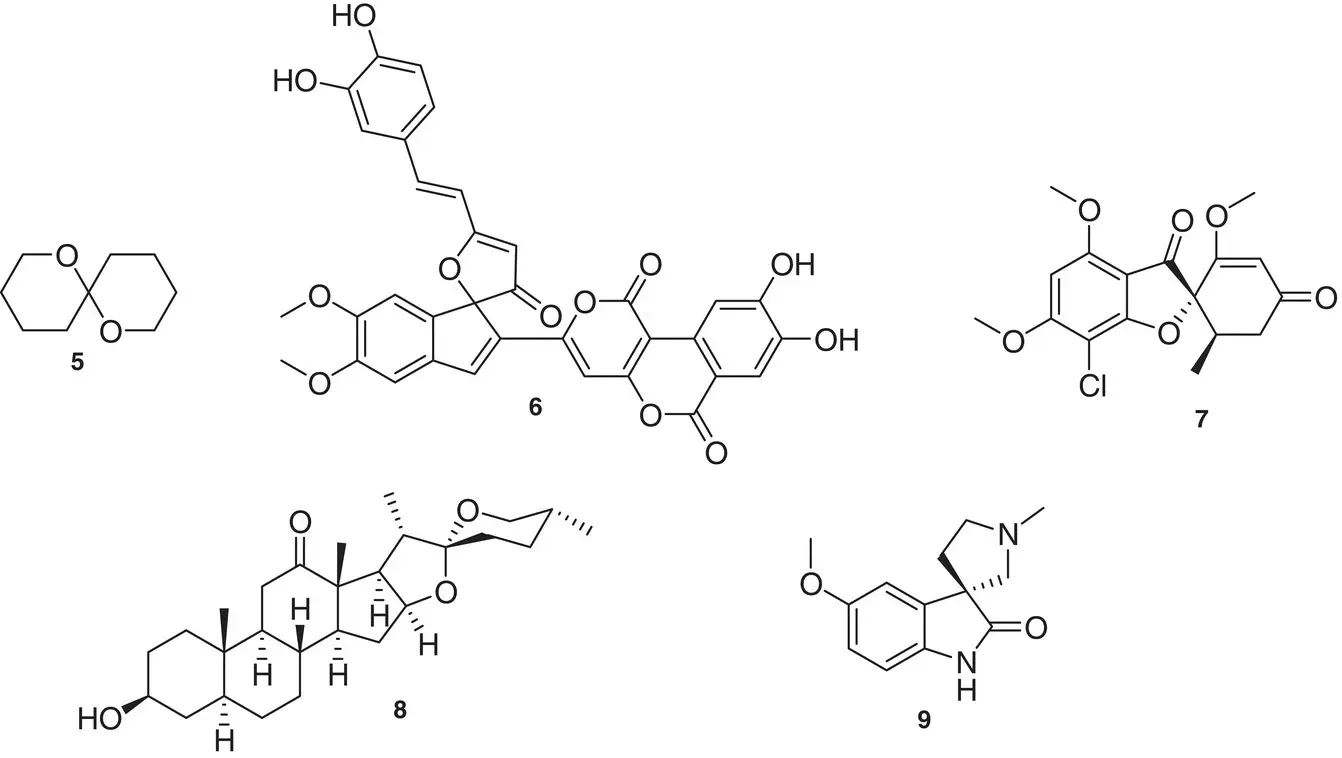
Figure 1.4 Examples of naturally occurring compounds containing the spiro moiety.
A classic example of the importance of the presence of a spiro functionality is the retention of the biological activity of perhydrohistrionicotoxin 10, the completely reduced analogue of the potent nicotinic receptor antagonist alkaloid (−)‐histrionicotoxin 11, isolated from “dart‐poison” frogs, that clearly suggests the fundamental role of the spiropiperidine moiety in determining a strong receptor binding. The massive synthetic efforts on this topic are collected in a book chapter [12] ( Figure 1.5).
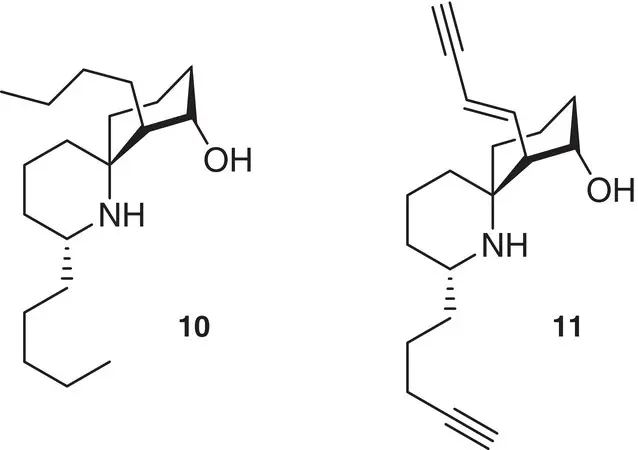
Figure 1.5 Spiro functionality in nicotinic receptor antagonists.
Source: Hart [12].
As stated before, spirocycles are present in successfully developed medications and represent attractive synthetic targets included in chemical libraries for diversity‐oriented synthesis within drug discovery projects. In this context, the spiro moiety has been and can be employed both as core structure and as an activity modulator, appended to decorate the peripheral part of the molecule [13].
The major advantage of spirocycles in biological applications as core structure or pharmacophores originates from their 3‐D nature and the associated conformational features that allow for a better ability to interact with the target protein enzyme. The tetrahedral feature of the spiro atom renders the two ring planes nearly perpendicular to each other with a limited number of potential conformations. When added in the periphery of the molecule, the spirocycle acts as a modulator of physicochemical properties such as log P and water solubility, as well as affecting the metabolic stability of the molecule. Not least, from an intellectual property perspective, the introduction of spirocycles offers the possibility of obtaining a free patent space in a me‐too research approach.
Prominent examples of marketed spirocompounds, illustrating these concepts, include fluspirilene 12, spiraprilat 13,and cevimeline 14, while experimental compounds in different stages of clinical development are ETX0914 15,a DNA gyrase inhibitor; tofoglifozin CSG452 16,an inhibitor of hSGLT2 for the treatment of Type 2 diabetes; AZD1979 17,an antagonist of melanin‐concentrating hormone receptor; and rolapitant 18,a neurokinine 1 receptor antagonist [13, 14] ( Figure 1.6).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.