Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
2.2 General Features
The sp 3‐rich character of spirocycles and resulting extended range of exit vectors spanning across all three spatial dimensions represent an exciting opportunity for expanding molecular complexity while maintaining structural rigidity. These two parameters are also of prime importance for optimizing the thermodynamic fingerprint of chemical probes and drugs, and achieve optimal potency and selectivity profiles. At the same time, structural novelty opens valuable avenues for exploring new chemical spaces and IP protection of novel bioactive scaffolds. Spirocycles give access to more rigid scaffolds and better‐defined directionality of exit vectors, particularly for spirocycles containing small rings (e.g. cyclobutanes, oxetanes, azetidines, and thietanes, Figures 2.2and 2.3), which are conformationally highly restricted. Studies by Lovering showed a positive correlation between the simple indicator of saturation Fsp 3= (number of sp 3hybridized carbons/total carbon count) and the number of stereogenic centers as compounds progress through clinical trials, suggesting an enrichment in these features along clinical development [26]. Another direct consequence of their sp 3richness is their higher density of exit vectors ( D ev, Figure 2.2) compared with sp 2‐rich systems. D evcan be a useful primary indicator to rapidly estimate the three‐dimensionality of a scaffold and its potential for molecular complexity and diversity generation through synthesis. We use this indicator regularly in our lab as a criterion to prioritize fragment hit‐to‐lead optimization. This contrasts with some scaffolds that have been at the center of drug discovery over the last decades. Those include the typical poly(hetero)aromatic structures which result from linking of multiple two‐dimensional units, and provide limited scope for targeting the three dimensions. As a result, the 3D character of spirocycles and greatly extended range of accessible vectorizations are regarded as powerful ways to “escape from flatland” [26, 27] through the bioisosteric replacement of “flat”, sp 2‐rich scaffolds found in historical fragment and lead screening libraries.
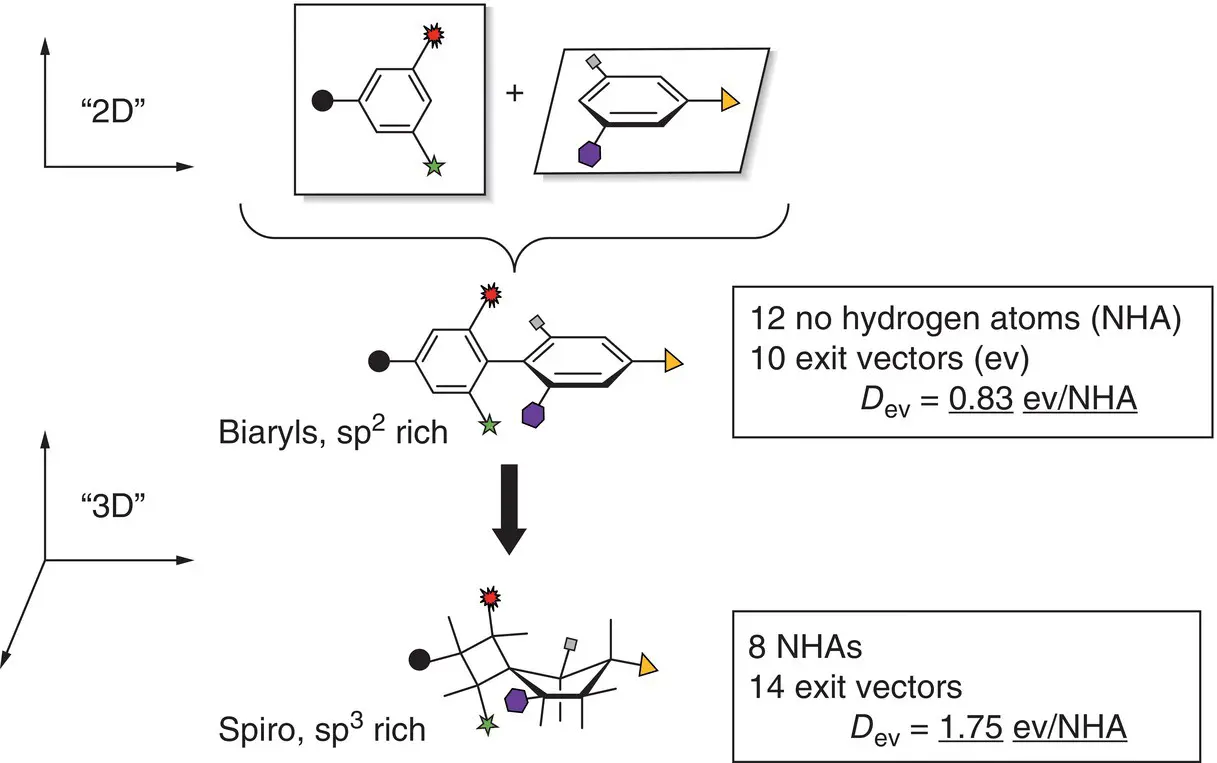
Figure 2.2 Schematic comparison of substituted biaryl and spiro[3,4]octane scaffolds: the overall conservation of the relative orientation and high density of exit vectors can be exploited for bioisosteric replacement strategies.
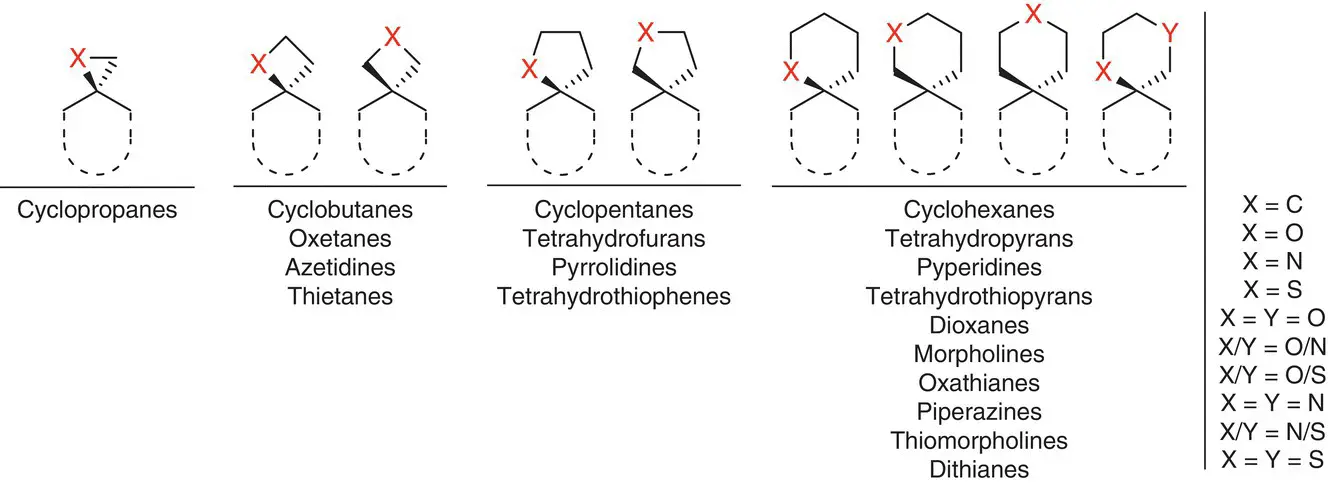
Figure 2.3 Examples of saturated spirocyclic ring systems frequently encountered in drug discovery.
The constrained and directional exit vectors projected from “spiro” scaffolds allow fine‐tuning the relative orientations of functional groups within bioactive molecules in order to achieve optimal molecular interaction with a target receptor. Such rigidity can also be exploited for the bioisosteric replacement and conformational restriction of flexible moieties in biomolecules, hence reducing detrimental entropic penalties for binding [28]. Spirocycles have also been used to modulate physicochemical properties and pharmacokinetics of biomolecules, including aqueous solubility and metabolic stability [26, 27, 29]. A number of reports have also highlighted that a high sp 2/aromatic content is generally detrimental to the physicochemical properties of drug candidates [26, 27, 30, 31].
2.3 Property Optimization in Bioactive Compounds
Spirocyclic structures displaying a wide range of biological activities have been reported [32, 33] ( Figure 2.4). For example, the representative SAR405838 ( 16) belongs to a family of potent spirooxindole derivatives potently inhibiting MDM2 [34, 35]. MDM2 inhibition has attracted high attention for the development of anticancer therapeutics acting via reactivation of the tumor suppressor protein p53. The oxindole ring mimics the Trp23 side chain of p53, and makes key contacts with Leu54 backbone carbonyl in MDM2 via its NH group (protein data bank codes 1YCR and 3LBL). At the same time, the spirooxindole motif provides conformational restriction to the scaffold, “locking” the molecule in the optimal conformation for binding MDM2 with sub‐nanomolar affinity. 16was recently examined in phase 1 clinical trials in patients with advanced solid tumors [36, 37]. Conformational restriction has also previously been employed by Schafmeister and coworkers for the development of covalently constrained helix mimetics targeting MDM2 [38]. Solid‐phase synthesis of the peptidic backbone followed by formation of the diketopiperazine linkages led to the rigid spiro‐oligomer 17( K d= 400 nM) [38–40], whose hydrophobic R‐groups project in a (i, i+4, i+8) fashion. Those mimic the side chains of residues Phe19, Trp23, and Leu26 of p53 and make key contacts at the MDM2 surface. 17displayed cell permeability and activity, and induced p53 upregulation in Huh7 cells. It is worth noting that the backbone of these constrained helix mimetics have very low conformational freedom, making their de novo design challenging and as a result, only few examples have been reported. Another good example of spirocycle functionalized compound is the Calcitonin Gene‐Related Peptide (CGRP) Receptor antagonist 18, which has progressed to Phase 3 clinical trials and is expected to reach the market for the treatment of migraine in the early 2020s [41]. Introduction of the spirocycle in this molecule led to enhanced metabolic stability compared with non‐spirocyclic analogues, for example, bearing labile and oxidation prone dihydroquinazolinone units [42]. Lead molecule 19was discovered as part of a lead optimization effort at AstraZeneca aimed at improving the properties of molecules targeting the central nervous system (CNS), more precisely the melanin‐concentrating hormone receptor 1 (MCHr1), a target protein for the treatment of obesity and its downstream effects [43]. An important focus has been to identify a chemical series with attractive physicochemical properties, including reduced interaction with the ether‐a‐go‐go‐related gene (hERG) channel [44] while retaining fine‐tuned lipophilicity profiles and sufficient CNS exposure [45]. The appended spirocyclic moiety provided improved physicochemical properties required for CNS exposure while lowering off‐target interactions toward hERG, compared with morpholine and open ring analogues. Another early example is the triaryl bis‐sulfone derivative 20, which was identified by Merck as a potent type 2 canabinoid receptor (CB2) antagonist [46]. Modulation of CB2 has been proposed as a strategy for the development of immunomodulators. Replacement of the central phenyl ring of 20with a spirocyclopropyl piperidine, saturated unit led to sulphonamide analogue 21. The latter displayed a similar binding mode, potency, and selectivity profile against CB2 as parent 20. Reduction in the number of aromatic rings is known to affect the physicochemical properties, hence developability of leads [31, 47]. Pleasingly, 21showed reduced off‐target effects as exemplified by a c. 10‐fold reduction in affinity against the rat calcium channel and the cytochrome P450 2C9, while also presenting a beneficial clogP drop from 2.81 to 1.48.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.