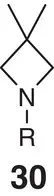Another illustrative successful example is the bioisosteric replacement of the metabolically labile morpholine unit of BTK inhibitor 32( Figure 2.7). This led to the development of 2‐oxa‐6‐azaspiro[3.3]heptane functionalized analogue 33, which displays enhanced stability and solubility while retaining similar potency to its target compared with the parent molecule [57].
Thalidomide 34( Figure 2.8) was introduced on the market in the late 1950s–early 1960s as an antiemetic and sedative, and banned a few years later due to its shattering side‐effects. Overall, thalidomide is thought to have caused numerous and severe birth defects in more than 10 000 children during that time [25]. While thalidomide was initially administered as a racemic mixture, it has since been shown that the ( R ) enantiomer has sedative effects and the ( S ) enantiomer is teratogenic. It is also known that racemization spontaneously takes place in vivo due to the acidity of H a( Figure 2.8), although as of today the exact mechanism underlying the teratogenicity of thalidomide is still under investigation. In 2013, Carreira and coworkers reported derivative 35where one of the imide carbonyls is replaced by an oxetane [56], with the goal of limiting in vivo metabolism and racemization, and potentially altering teratogenicity ( Figure 2.8). Not unexpectedly, 35displayed higher p K a, lower lipophilicity, and increased solubility. It displayed similar intrinsic clearance rates in human microsomes compared with 34; however, showed much improved plasma stability after five hours. Pleasingly, 35was configurationally stable to racemization in human blood plasma after five hours, thereby showing that a carbonyl to oxetane switch can be a viable approach to mitigate epimerization at adjacent stereocenters.
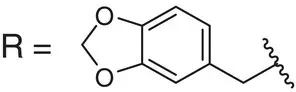
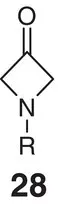
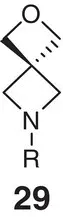
Table 2.2 Physicochemical and biochemical properties of selected oxetanes, and their carbonylated and gem‐dimethyl counterparts.
Source: Based on Wuitschik et al. [55].
 |
 |
 |
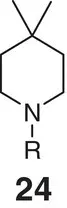 |
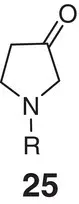 |
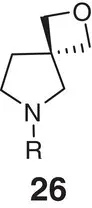 |
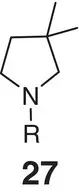 |
 |
 |
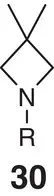 |
| Solubility (μg ml −1) |
4000 |
1400 |
220 |
4100 |
730 |
40 |
n.d. |
24 000 |
290 |
| Lipophilicity logD (logP) |
1.2 (1.6) |
1.0 (2.0) |
2.3 (4.4) |
−0.1 (−0.1) |
0.7 (1.5) |
1.4 (3.7) |
n.d. |
0.5 (1.2) |
0.8 (3.1) |
| hCL int(min −1mg −1μl) |
120 |
6 |
23 |
100 |
2 |
10 |
n.d. |
3 |
0 |
| mCL int(min −1mg −1μl) |
88 |
22 |
31 |
580 |
27 |
39 |
n.d. |
7 |
16 |
| p K aamine |
7.5 |
8.3 |
9.5 |
6.1 |
8.1 |
9.7 |
n.d. |
8.0 |
9.6 |
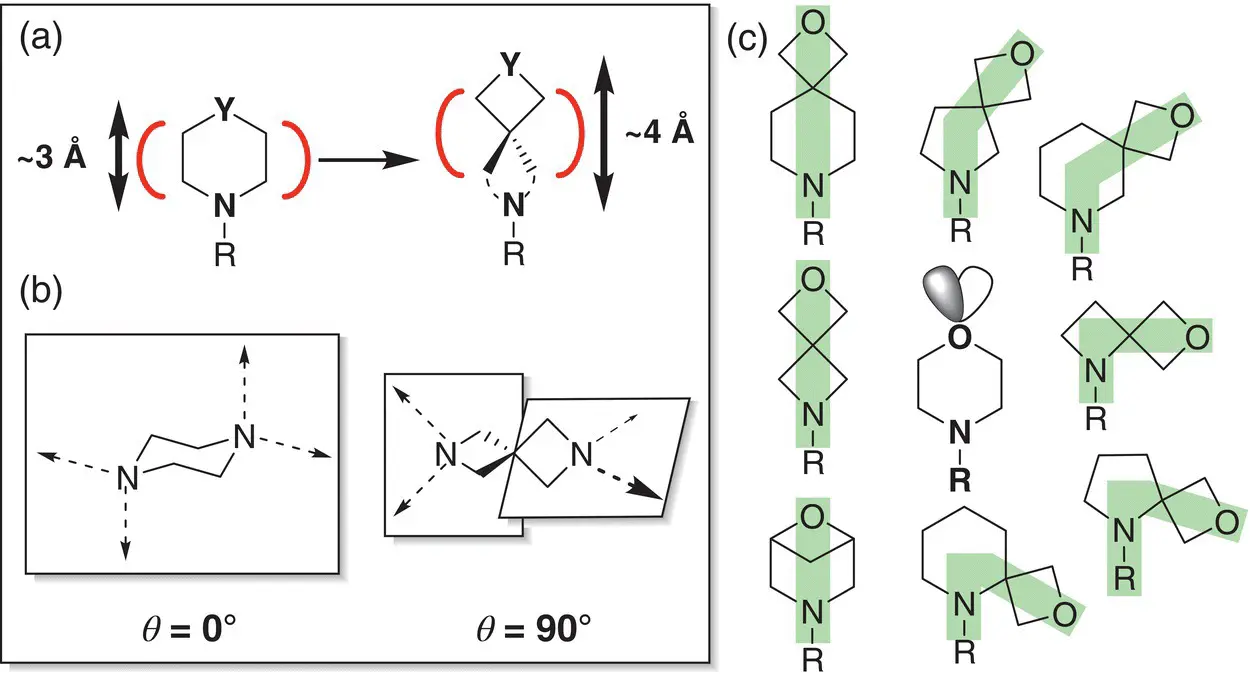
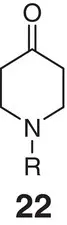
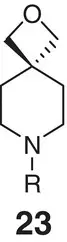
Figure 2.6 (a) Spiro‐oxetane as a morpholine bioisostere.
Sources: Based on Carreira and Fessard [48]; Burkhard et al. [56];
(b) comparison of exit vectors in the piperidine motif and its spiro counterpart; (c) spirocyclic oxetane based on mimics of morpholine.
Table 2.3 Comparison of the properties of spirocyclic oxetanes with their morpholine parent.
Source: Adapted from Carreira Wuitschik et al. [55].
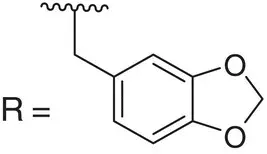 |
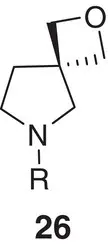 |
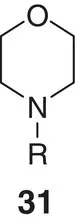 |
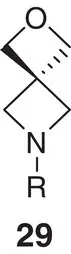 |
| Solubility (μg ml −1) |
730 |
8000 |
24 000 |
| Lipophilicity logD (logP) |
0.7 (1.5) |
1.5 (1.6) |
0.5 (1.2) |
| hCL int(min −1mg −1μl) |
2 |
9 |
3 |
| mCL int(min −1mg −1μl) |
27 |
8 |
7 |
| p K aamine |
8.1 |
7.0 |
8.0 |
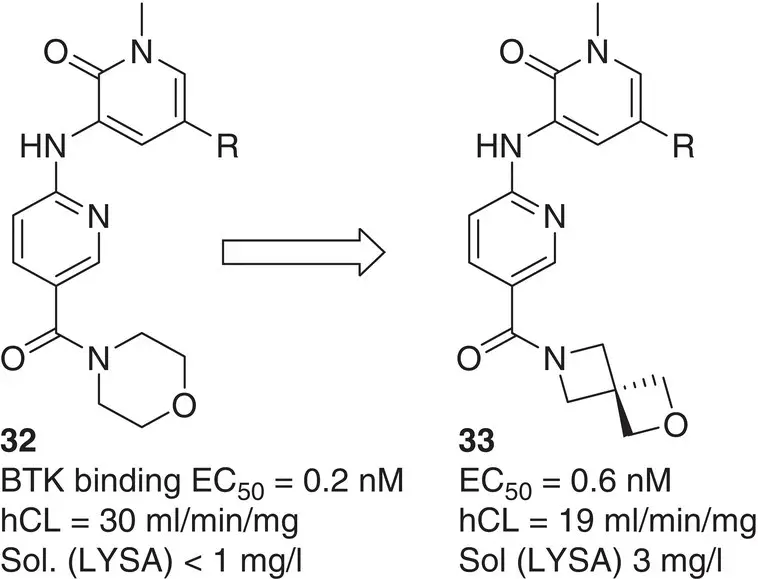
Figure 2.7 Replacement of morpholine by 2‐oxa‐6‐azaspiro[3.3]heptane in BTK inhibitors.
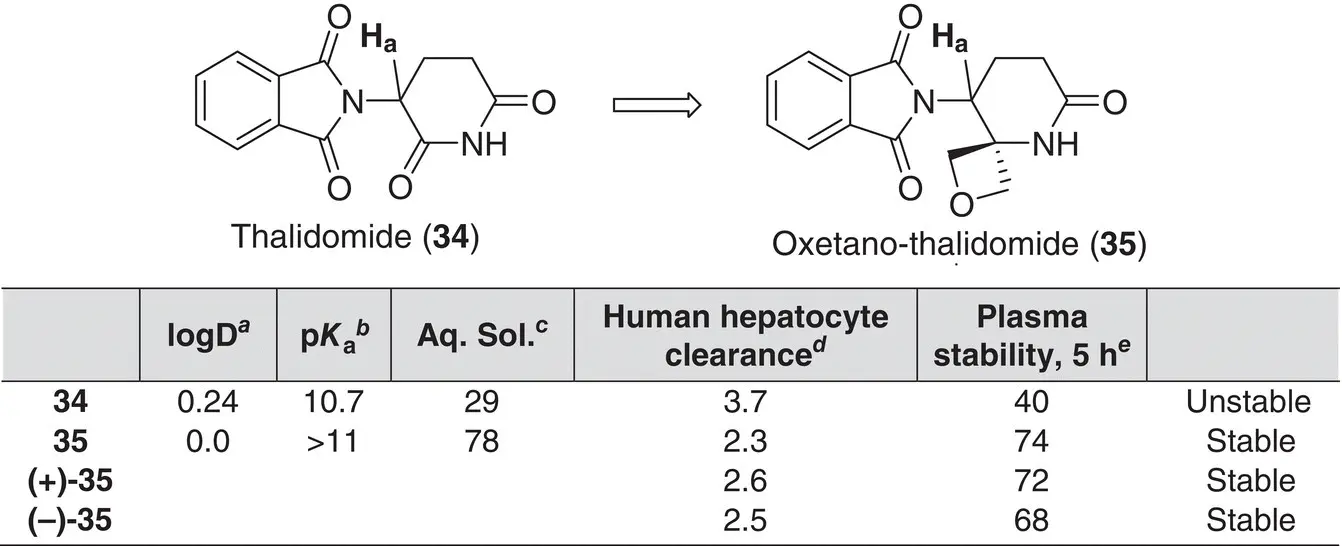
Figure 2.8 Top: Structure of thalidomide 34and its oxetano analogue 35. Bottom: comparison of the physicochemical properties of thalidomide and oxetano‐thalidomide.
Source: Modified from Burkhard et al. [56].
Notes: aLogarithmic n‐octanol/water distribution coefficient at pH 7.4. bIonization constants determined at 23 °C by spectrophotometry in water. cIntrinsic solubility measured at pH 6.5 by a Lyophilization Solubility Assay [μg ml −1]. dIntrinsic clearance rates [μl min −110 −6cells] measured in human hepatocytes. eStability in human plasma after five hours incubation time, expressed as a percentage of the initial concentration. The reported values represent the average of three runs ( n = 3).
Читать дальше