Lebedev (1940) studied the physicochemical transformations in glass in the region of T g, which depends on chemical composition and thermal history. He demonstrated that the toughened state of silicate glasses is not only characterized by the existence of internal stress, but is also distinguished by its structure. According to him, the difference between the refractive indices of toughened and annealed glasses could not be attributed only to the existence of internal stresses in thermally tempered glass. Lebedev observed (Evstropyev 1953, p. 10) that the refractive index ( n ) of a silicate glass increases linearly with the increase in temperature ( T ) up to about 500 °C and then decreases rapidly between 520 and 590 °C. He also noticed that the linear section of the n – T plot (i.e. below 500 °C) was “rapidly” reversible and thus purely a thermal effect. However, if the glass is heated to high temperatures (say 600 °C) and then cooled, the descending branch of the n – T curve is not “rapidly” reversible, meaning – if not soaked for very long times at each step of cooling. This means refractive index of glass and hence the structure of glass depends on thermal history.
After considering the limitations of X‐ray analysis and techniques available at that time, Lebedev (1940) concluded that it was necessary to combine the X‐ray structural analysis with other methods, both physical and chemical, unlike the approach taken by Zachariasen (1932) and supported by Warren (1940) entirely on the basis of X‐ray analysis. In those days, of course, the modern techniques such as small‐angle X‐ray scattering and wide‐angle X‐ray scattering, nuclear magnetic resonance, X‐ray absorption fine structure, and neutron scattering were not available.
Chemically, quartz is SiO 2, but the crystals exist in two crystalline forms, α‐quartz at normal temperatures and β‐quartz at high temperatures. The transformation from α‐quartz to β‐quartz takes place at about 573 °C (846 K). This transformation is accompanied by significant changes in volume, and hence density and other physical properties. Because of this α–β transition in crystalline quartz, Lebedev (1940) postulated that the sharp decrease in the refractive index of a silicate glass in the range 520–590 °C, mentioned above, is associated with the α–β transition in quartz and thus the microcrystalline formations, one form of which consists of quartz microcrystals. Real silicate glass may be regarded as a conglomeration of “sub‐microcrystalline” formations of various types of silicates and silica, the chemical nature of which depends on the chemical composition of the glass. The term “sub‐microcrystalline” is used to distinguish them from fragments of microcrystals with a perfect regular lattice. A new name was coined: “crystallites.” The greatest regularity in the lattice structure is thought to be in the central regions of the crystallites. The deviation of the crystal lattice occurs as the periphery of the crystallites is approached. The crystallites are separated by a mass of amorphous medium in which the degree of disorder in the atomic arrangements increases with increasing distance from the crystalline regions. The crystallites may have definite chemical compounds or they could be solid solution, the nature of which depends on the phase diagrams for the system that constitutes the glass.
2.4.3 Composition of Standard Glass
Ward‐Harvey (2009) dates the glass industry back to ancient Egypt – more than 3500 years ago. Yet, even today we are making advances in chemical composition and processing that are enabling the growth of technology, such as flexible glasses for displays.
As mentioned in Section 2.2.2, in oxide glasses, the oxygen atoms form bridges and network formers, such as silicon, boron, phosphorus, or germanium, form strong bonds with them in a randomly arranged network structure. Network modifiers, such as sodium and calcium, generally sit in ionic form within interstitial holes. Such ions and intermediates are added to optimize the properties of glass for specific functions and/or aesthetics. A striking example is the addition of small amounts of specific metal ions to add color to glass: manganese for purple, iron for green, copper for blue, etc. The glass constituents play a role not only in the final product, but also during the production process. Fluxes made from sodium or potassium carbonate can lower the melting point and glass transition temperature of the formers while stabilizers, such as calcium oxide, provide water/humidity resistance to intermediates, such as sodium silicate.
Silicon ions are the most common network formers, but pure silicate glasses can be hard to work due to quartz's high melting temperature (1996 K). Soda‐lime glasses (originally Na 2O (sodium oxide) + CaO (lime) but also typically includes MgO (magnesia) and Al 2O 3(alumina)) are thus the norm for multiple uses, such as windows and jars. Borosilicate glasses, which can include 5–13% boron trioxide, are less vulnerable to thermal shock and are used in cookware and labware (e.g. Pyrex). Aluminosilicate glasses (5–10% alumina) also have good thermal resistance, but are harder to shape and so are used for purposes such as fiberglass. Phosphate glasses (phosphorus pentoxide) have been found to be compatible with the organic mineral phase of bone and are finding increasing biomedical uses (Rahaman 2014).
Modern glasses can have quite a complex chemical composition and the final structure is made even more complex by the potential bonding arrangements. Figure 2.8shows the composition of a typical container glass examined by X‐ray fluorescence by Hsich (1980).
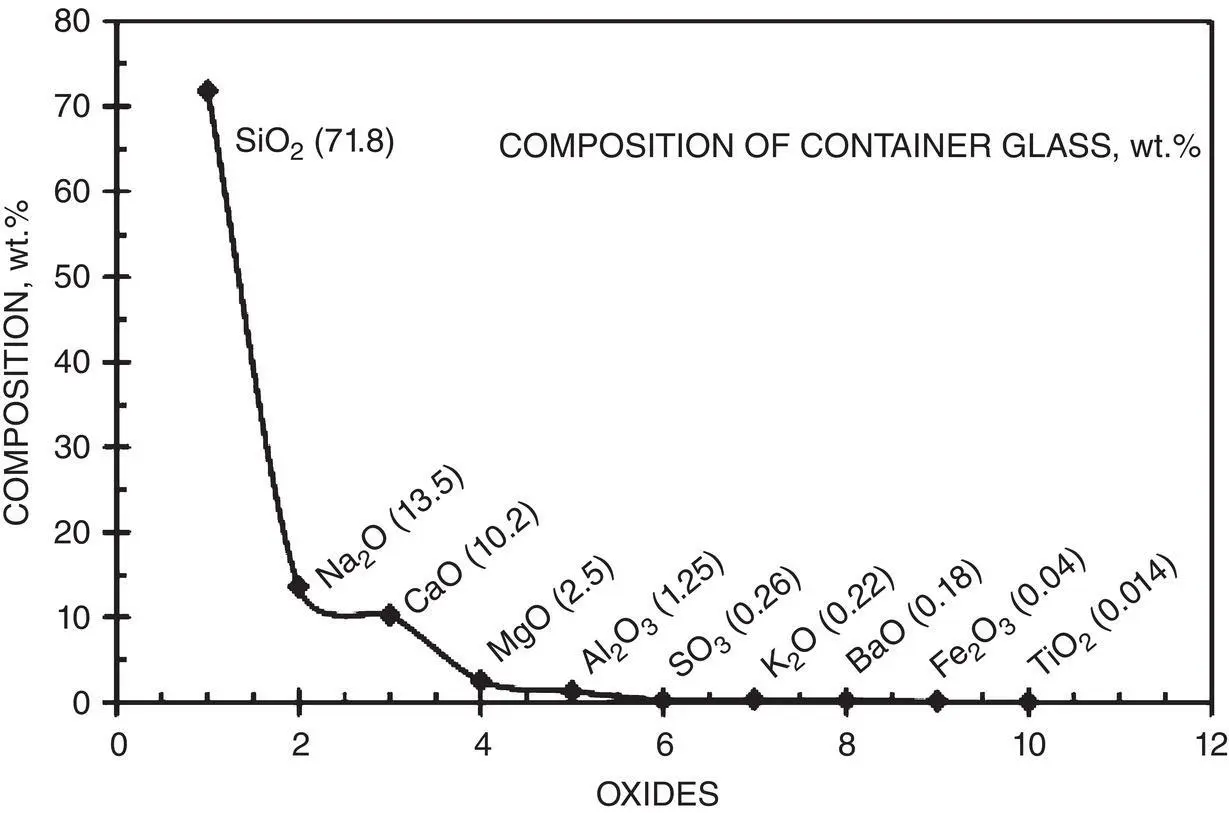
Figure 2.8 Composition of a typical soda‐lime container glass given in weight percent.
Source: Modified from Hsich (1980).
The composition of glass can also be modified post initial hardening. For example, the surface of Corning's well‐known Gorilla glass – used in a variety of smart phones and devices – is toughened by a process called ion exchange (Corning n.d.). The material is immersed in a molten alkaline potassium salt causing smaller sodium ions in the glass to be replaced by larger potassium ions from the bath. The larger ions create a surface layer with high residual compressive stress and increase the surface's resistance to damage. However, the full magic to Gorilla glass comes from controlling the stresses at the surface and throughout the center of the glass through its forming processes (Bushwick 2013).
The resistance to weathering, scratching, and breaking of glass plates is significantly improved by a single and simple process called thermal tempering (Lebedev 1912). When a tempered or toughened glass sheet breaks, it shatters into small fragments that are less harmful than the sharp‐edged large pieces of glass into which ordinary annealed glass generally breaks. Thus, the safety aspects of doors and windows are improved immensely. Highly compressed surface layers of toughened sheets of glass also improve the strength and durability. For a given thickness, the shape and size of fragment after fracturing depend on the tensile stress in the middle. The stress distribution is parabolic with the maximum tension in the middle plane and about half of the compressive stress at the surfaces. The change in the engineering properties of tempered glass is due to not only mechanical stresses, but also structural changes within the glass plate. These changes in the structure also affect optical properties, as illustrated in Figure 2.9.
Читать дальше













