High-Performance Materials from Bio-based Feedstocks
Здесь есть возможность читать онлайн «High-Performance Materials from Bio-based Feedstocks» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:High-Performance Materials from Bio-based Feedstocks
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
High-Performance Materials from Bio-based Feedstocks: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «High-Performance Materials from Bio-based Feedstocks»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
High-Performance Materials from Bio-based Feedstocks
The latest advancements in the production, properties, and performance of bio-based feedstock materials
www.wiley.com/go/rrs High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «High-Performance Materials from Bio-based Feedstocks», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
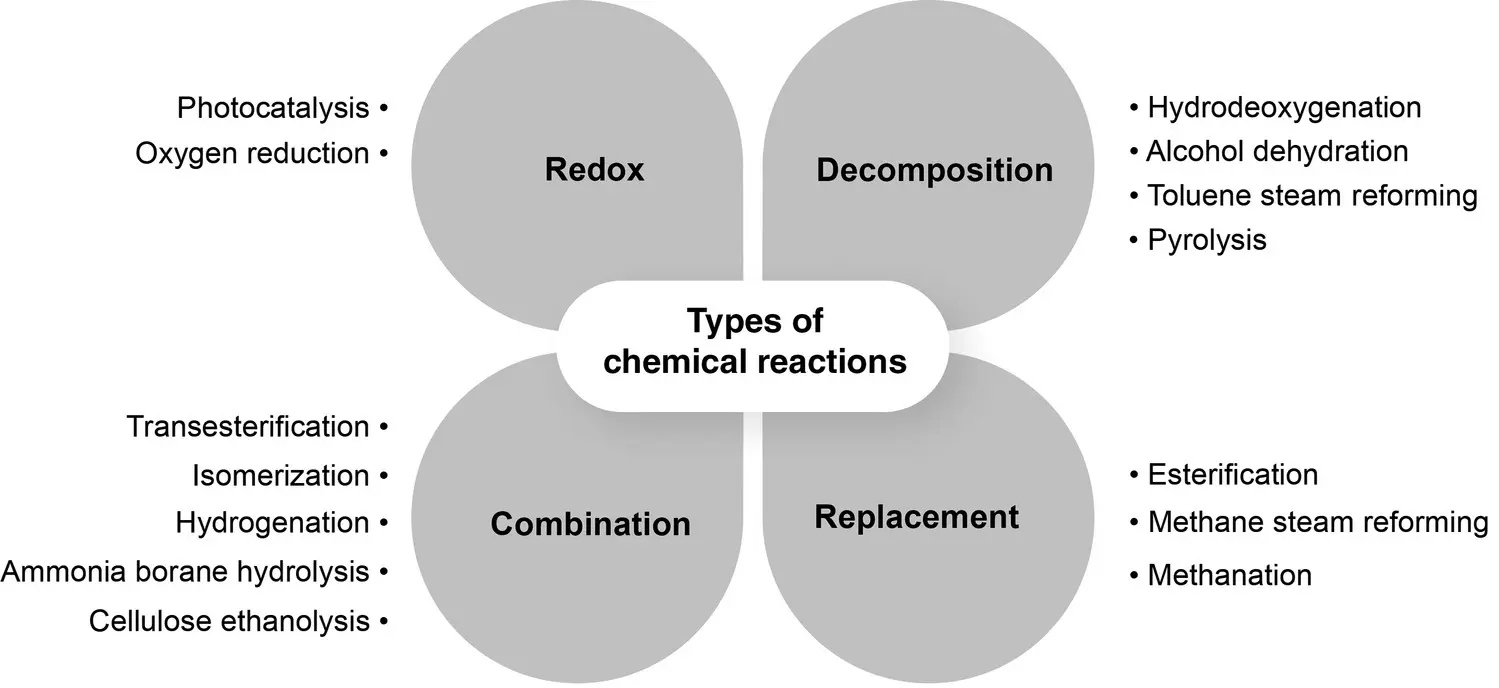
Figure 2.4 Examples of chemical reactions catalyzed by biomass‐derived carbons.
The existence of organic compounds in the renewable resource matrix, particularly animal wastes, brings about occurring minerals and inorganic alkalis in the structure of the produced biochar, such as K, Ca, Mg, N, P, and S [85–88]. These elements may be present in the form of chemical compounds such as CaCO 3, KCl, or SiCl 4[8]. These minerals and inorganic alkalis can behave like a natural promoter of biochar activity in some catalyzed reactions. For example, the alkali and alkali earth metallic species could markedly promote the catalytic activity of biochar in tar reforming during biomass gasification [89]. An increasing biomass pyrolysis temperature resulted in enhanced fixed carbon and mineral contents in the produced biochar [83]. However, the number of surface functional groups within the biochar decreased with increasing carbonization temperatures because hetero‐atomic functional groups containing such oxygen and nitrogen atoms were volatilized from the biomass structure [90]. The remaining elements in the biomass after the volatile compounds’ detachment rearranged themselves to form more aromatic structures, causing a reduction in the number of active sites.
2.5.2 Modified Biochar
To develop the physicochemical properties and the number of active species on biochar toward a higher activity and maximum catalytic performance, a large number of modification techniques have been investigated including metal modifications as well as chemical and physical treatments.
2.5.2.1 Tar‐reforming Processes
The production of syngas via biomass gasification has attracted a great deal of interest. However, this technology faces some challenges, the biggest one of which is excessive tar formation resulting in clogged up equipment. Consequently, the total cost of biomass‐derived syngas production is increased making it difficult to develop this process into industrial manufacturing. To overcome this drawback, tar removal technologies have been intensively researched with regard to their economic and environmental impacts. The catalytic thermochemical conversion using biochar catalysts has been reported as a capable technique for tar reforming, but currently still shows inferior performance compared to conventional metal‐supported catalysts. Particularly steam and CO 2‐treated biochars are very powerful catalysts for tar reforming. The biochar prepared by pyrolysis from several biomass sources was activated with 15 vol% H 2O mixed with argon or with CO 2at 800 °C for a short time. The treated biochar catalysts increased the catalytic activity in the steam or CO 2reforming of tar compared to a regular biochar. Treatment of the biochar resulted in an increased surface area and pore volume (both microporous and mesoporous), and high content of oxygenated functional groups [91, 92]. The CO 2generated more micropores in the biochar, whereas conversely, steam created more mesopores, which adds further importance for tar reforming. Even though micropores showed a greater initial tar conversion, these are rapidly deactivated due to coke deposition in the pores [4, 91, 93]. The steam‐activated biochar had more oxygenated functional groups in the aromatic C–O forms, which are more active sites for tar reforming, than those treated by CO 2[89, 92, 94]. Tar molecules were probably absorbed onto the unstable aromatic C–O structures on the biochar surface bringing about the transformation of tar into the gas products. When comparing the reforming gases, the treated biochar catalyzing the tar steam reforming was more effective than that in the CO 2reforming [95]. The steam fed during the reforming reaction could produce additional oxygenated functional groups and aromatic C–O structures [92], which resulted in a gradual increase in catalytic performance. Therefore, steam was a promising agent for boosting and preserving the catalytic activity of biochar in tar reforming.
As discussed previously, raw biochar had slower rates of tar elimination than metal‐supported catalysts. Doping an active metal such as Ni and Fe onto the biochar surface is another favored approach for the development of catalytic biochar [96]. Kastner et al. prepared biochar by pyrolysis of pine bark, which was then impregnated with Fe [97]. Compared to the regular biochar, the Fe‐modified biochar catalyst possessed a lower surface area and pore volume as the Fe particles could hinder and shrink the biochar pores. Although the physical properties of the Fe‐modified biochar catalyst were not remarkable, this catalyst showed that the tar decomposition rate was increased and the activation energy was decreased by 47%. The tar conversion was significantly altered by the metal loading and the tar decomposition increased with higher metal content on the biochar. At a decomposition temperature of 900 °C, the biochar modified with 13% Fe had a tar conversion of 100%. The addition of alkali along with the metal impregnation on biochar was developed in order to further enhance the catalytic activity. The raw biomass material was impregnated with potassium ferrate prior to carbonization at 900 °C for two hours to obtain a K–Fe bimetallic catalyst‐supported biochar [98]. The K 2CO 3was formed as an active site of this catalyst and showed a superior catalytic activity in cracking of biomass pyrolysis tar at a relatively low reaction temperature (600–700 °C). The active metal sites and pore structure were still maintained after the reaction. This catalyst also showed a high stability as no significant change in tar conversion was observed after five cycles.
2.5.2.2 Biodiesel Production Processes
Biodiesel, a fuel derived from renewable sources such as vegetable oils and animal fats, has received much attention due to the continuous reduction in petroleum reserves and environmental issues. Biodiesel production via transesterification ( Figure 2.5), also known as alcoholysis, is currently the most attractive approach, and can be divided into non‐catalytic, biocatalytic, and chemical catalytic processes. The non‐catalytic process or supercritical alcohol process is carried out in conditions above critical temperature and pressure of the reaction mixture determined from the critical properties of alcohols and triglycerides. This process requires a relatively high reaction temperature of 230–450 °C and high pressure of 19–60 MPa, as well as excess methanol (molar ratio of oil to methanol approximately 1 : 40). These requirements are a limitation to the non‐catalytic process for biodiesel production on an industrial scale [99]. A biocatalytic or enzymatic process produces biofuel with a low environmental impact and can be performed at mild temperature and pressure. Such processes are also not sensitive to the free fatty acid and water content in the feedstock [100, 101]. However, enzyme stability, enzyme reuse, and the high cost of enzyme immobilization are the major drawbacks of this process. In conventional biodiesel production, chemical catalysts (both acidic and basic) are usually used. Solid catalysts have been widely applied in biodiesel production owing to their ease of separation from products and excess reactants. A number of research studies have been conducted on biodiesel production using various types of acidic and basic solid catalysts. Most common is the solid base catalyst or alkali catalyst that can catalyze transesterification reaction in even milder reaction conditions and shorter reaction time than acidic solid catalysts. Calcium oxide (CaO) solid base catalyst can be derived from calcium carbonate‐rich materials such as horn shell and eggshell. With the basic catalyst produced from calcined eggshell, the biodiesel yield reached 97% in transesterification of waste cooking oil and methanol at the ratio of 1 : 6 [102]. Nevertheless, the preparation of a solid base catalyst from calcium carbonate‐rich feedstocks requires high temperatures of 800–900 °C. In addition, the free fatty acid and moisture contents in the oil feedstock should be considered for alkali catalysts. Water molecules in the feedstock can hydrolyze triglycerides into diglycerides and monoglycerides, which yield a greater amount of free fatty acids. The alkali catalyst is able to convert free fatty acids into soap via the saponification side‐reaction. The free fatty acid in the feedstock should be lower than 2% for transesterification.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «High-Performance Materials from Bio-based Feedstocks»
Представляем Вашему вниманию похожие книги на «High-Performance Materials from Bio-based Feedstocks» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «High-Performance Materials from Bio-based Feedstocks» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












