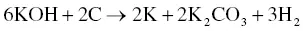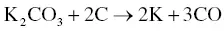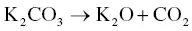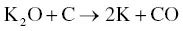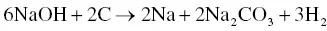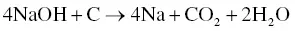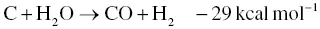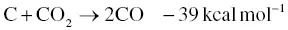2.3.2.1 Chemical Activation
The utilization of agricultural material as a raw material for the production of activated carbon is usually carried out by chemical activation. The proper temperature for chemical activation should be 400–600 °C. As the chemical activating agent should penetrate and erode the cellulose and lignin and destroy their structures during carbonization, the property of the activating agent should be strongly corrosive. Not only strong acidic and basic agents but also other strong oxidizing agents have been exploited. The most widely used chemical activating agents are potassium hydroxide (KOH), phosphoric acid (H 3PO 4) ,and zinc chloride (ZnCl 2) [45, 47], but chemical agents such as sodium chloride (NaCl), sodium hydroxide (NaOH), hydrogen peroxide (H 2O 2), and potassium carbonate (K 2CO 3) have also been used [48–50]. The chemical activating agents in an aqueous form are typically applied onto the raw materials via impregnation for a desired period of time. The duration of the chemical activation is often around 60–120 minutes, and the ratio of chemical activating agent and raw material is usually between 1 : 1 and 5 : 1. The reaction pathways when using KOH as an activating agent are presented in Eqs. (2.3)– (2.6)as follows [26]:
(2.3) 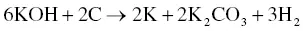
(2.4) 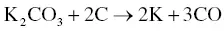
(2.5) 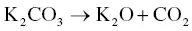
(2.6) 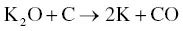
The activated carbon produced from soybean pods activated by KOH showed the highest surface area of 2245 m 2g −1[47]. An interesting shortcut in the reaction pathways was taken by employing K 2CO 3as an activating agent instead of KOH [48]. The K 2CO 3is an initial substance in Eq. (2.4), thus Eq. (2.3)could be eliminated. The activated carbon produced by using K 2CO 3also showed an extremely high surface area of 2613 m 2g −1along with a high pore volume of 1.66 cm 3g −1. However, the KOH showed a higher activation potential than the K 2CO 3at low carbonization temperatures with regard to the porosity and surface area of the resulting activated carbon [51], which was ascribed to the difference in melting point. The K 2CO 3has a higher melting temperature of 890 °C while the melting point of KOH is 360 °C. When using NaOH as an activating agent, the reaction pathways are presented in Eqs. (2.7)and (2.8)[52].
(2.7) 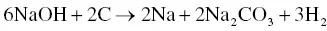
(2.8) 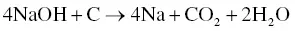
The reaction steps in heterogeneous catalysis will be discussed in detail in Section 2.4. Since most of the reactant adsorption onto the catalyst surface takes place within the micropores of activated carbon, the number and the dimension of micropores should be carefully considered. Macroporous and mesoporous structures of activated carbon provide the passageway of the reactant to the micropores, hence they impact the overall activity of a heterogeneous catalyst as well. Activated carbon obtained from the chemical activation process regularly presents a microporous or sub‐mesoporous structure with pore diameters in the range of 1–3 nm. Pretreatment of the raw material before activation showed a surprising result. The palm shell was soaked in 5 H 2SO 4for 24 hours and the excess H 2SO 4was removed by washing with deionized water. Finally, the treated palm shell was activated with H 3PO 4at 650 °C for two hours. The pore diameter of the obtained activated carbon was around 20 nm, which corresponds to a mesoporous structure. Furthermore, the proportion of each pore size can be controlled by the combination of several activating agents in one process [50]. Activation with NaCl resulted in activated carbon with a macroporous structure while NaOH provided micropores. The combination of NaCl and NaOH in the appropriate ratio thus enables control of the pore diameter.
Apart from the pore size and structure, also the chemical properties of activated carbon can be modified by chemical activation. The functional groups of activated carbon, especially oxygen‐containing groups, play an important role in catalysis. A variety of chemical activating agents affect the chemical properties of activated carbon. For example, NaOH activation of durian shell produced an activated carbon that contained a large amount of oxygen‐containing groups such as OH, C=O (ketone, aldehyde, lactones, and carboxyl), and C–O (anhydrides) [53]. In contrast, the oxygen‐containing functional groups disappeared from the surface of the activated carbon produced from Euphorbia rigida , which is an oil‐rich biomass [54]. Although ZnCl 2, K 2CO 3, NaOH, and H 3PO 4were used as activating agents, the produced activated carbon only contained hydrophobic groups (C–H, C–C, and C=C) [48]. As the hydrocarbon groups in the oil‐rich biomass could be cracked during the carbonization process, it generated more aromaticity of the activated carbon. Therefore, not only the type of chemical activating agent but also the category of biomass feedstock influence the chemical properties of activated carbon.
2.3.2.2 Physical Activation
For the development of the specific surface area and porous structure of activated carbon, the physical activation process is a favorable approach. Physical activation is achieved by carbonization in the presence of oxidizing gases such as steam, carbon dioxide (CO 2), or a mixture of these. This process resembles a partial gasification process which is generally carried out at temperatures between 700 and 1000 °C at atmospheric pressure. The furnace for the physical activation process is constructed with a gas flow unit, and a horizontal tube furnace or a fluidization system in a vertical tube are frequently selected [55, 56]. The oxygen in the activator gas molecule reacts with the carbon atom of biomass resulting in the generation of carbon monoxide (CO). The precise reaction pathways depend on the type of oxidizing gas and the activation temperature. Physical activation by steam and CO 2during carbonization occurs through the endothermic reactions as shown in Eqs. (2.9)and (2.10).
(2.9) 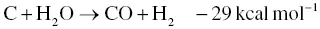
(2.10) 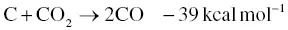
As previously mentioned, the physical activation is similar to partial gasification because the oxygen atoms of the oxidizing gas molecules can react with the carbon atom inside the biochar during activation. From the steam activation in Eq. (2.9), the carbon atom reacts with water molecule yielding CO, which can increase the porosity and surface area of activated carbon. Other prominent points of physical activation are to provide less contamination of activated carbon than chemical activation [57]. Physical activation does not require any neutralization procedure for the removal of the oxidizing agent. So, this process is more environmentally friendly. However, the use of fresh biomass or cellulosic material tends to produce ash during the physical activation process, which results in low activated carbon yields [3].
Читать дальше