High-Performance Materials from Bio-based Feedstocks
Здесь есть возможность читать онлайн «High-Performance Materials from Bio-based Feedstocks» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:High-Performance Materials from Bio-based Feedstocks
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
High-Performance Materials from Bio-based Feedstocks: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «High-Performance Materials from Bio-based Feedstocks»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
High-Performance Materials from Bio-based Feedstocks
The latest advancements in the production, properties, and performance of bio-based feedstock materials
www.wiley.com/go/rrs High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «High-Performance Materials from Bio-based Feedstocks», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The activation conditions are one of the important factors considered in promoting a high surface area and high product yield. The proper carbonization period is more influential on the final properties of activated carbon than the activation period. In the work of Rezma et al. [36], the biomass was first carbonized at 1000 °C and the resulting product was activated by CO 2at 750–900 °C for 30 minutes. The activated carbon presented a very low surface area and product yield [36]. As shown by Grima‐Olmedo et al. [19], the surface area of carbonized eucalyptus was improved when the temperature was raised from 600 to 800 °C. The addition of CO 2during carbonization strongly enhanced the surface area of the produced activated carbon [19].
Both micro‐ and mesopores can be generated through physical activation. For example, the micropores inside carbonized rubber wood sawdust could be changed into mesopores via steam activation, resulting in a high surface area of 1134 m 2g −1[58]. The surface functional groups of physically activated carbons correspond to those produced by chemical activation [39]. The factors that affect the physical and chemical properties of activated carbon can be prioritized as: (i) carbonization and activation temperatures, (ii) type of feedstock, (iii) particle size of feedstock, (iv) heating rate, (v) gas flow rate, and (vi) activation time [28]. This information is significant for the design of the physical activation process.
2.3.3 Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is an environmentally friendly thermochemical process for biomass conversion. This process operates at temperatures of 180–300 °C under the pressure of 8–20 bar in water [59]. The thermal processing at high pressure in water destructs the biomass structure and the resulting carbon material is called a hydrochar. The HTC is proposed as a productive process for fine biomass particles because it presents a higher yield of the bio‐based carbon product compared to regular carbonization [60]. Moreover, carbonization under high vapor pressure of water can produce hydrochar with uniform particles.
According to the hydrothermal reaction of the lignocellulosic material, the cellulose and hemicellulose are hydrolyzed to obtain CO 2as a by‐product according to Eq. (2.11).
(2.11) 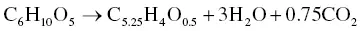
Carbon atoms do not appear in Eq. (2.11), which differs from the ordinary carbonization process shown in Eq. (2.1), as the transformation of biomass into hydrochar is essentially associated with three reactions: hydrolysis, dehydration, and decarboxylation [61]. However, the operating conditions of the HTC process are not able to destroy the lignin structure. Fourier‐transform infrared (FTIR) spectra showed the amount of lignin that was still present in the carbon material produced from the HTC process [62]. The HTC process is thus not appropriate for the conversion of lignin‐rich biomasses. Interestingly, the hydrothermal conversion of glucose (C 6H 12O 6) or other monosaccharides as seen in Eq. (2.12)could directly affect the carbon yield at 200 °C and 20 bar [27].
(2.12) 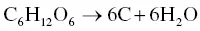
Even though hydrochars usually have a lower surface area than biochars, hydrochars possess a 1–4 times higher oxygen content than the carbon materials derived from carbonization and pyrolysis processes [33]. The hydrochars prepared from pinewood, peanut shells, or bamboo showed several oxygen‐containing groups such as C–O, –OH, and C=O, which were absent in the biochar [30]. The superior oxygen content in hydrochar makes it advantageous for many industrial sections such as catalysts or catalyst support. Nevertheless, an increase in hydrothermal temperature decreased the amount of oxygen and, conversely, the carbon content increased [11, 38]. Hydrochar is frequently used as a starting material for the production of activated carbon. The oxidizing agent can react with the oxygenated groups on the hydrochar surface resulting in its excellent catalytic behavior [63]. Moreover, the surface of hydrochar could be modified for specific applications. For example, hydrochar treated with sulfonic groups was effective in the production of biofuels [64].
2.3.4 Graphene Preparation from Biomass
Graphite is an infinite three‐dimensional (3D) material comprising several stacked layers anchored by weak Van der Waals forces. Each stacked layer is a two‐dimensional (2D) carbon sheet structured in a hexagonal honeycomb lattice called graphene [65, 66]. Graphene was discovered in 2004 and presents astonishing properties. Its appearance is like a soft film, and it has a high Young’s modulus and excellent thermal and electrical properties. Previous research reveals that graphene has a very high surface area of over 2000 m 2g −1and can be easily chemically modified [67]. Within the past decade, numerous investigations have used graphene in various applications along with exploring the practical synthesis procedures of graphene from biomass [68, 69]. Graphene can be synthesized through several methods including micromechanical exfoliation of graphite, chemical vapor deposition (CVD) of graphite, epitaxial graphene growth on silicon carbide, chemical graphitization of graphene oxide, and carbonization of biomass and waste materials [70–74]. Micromechanical exfoliation and CVD of graphite produced good‐quality graphene. The transformation of biomass into graphene can be accomplished via the carbonization of biomass under specific conditions. For example, pyrolysis of camphor leaves at an ultra‐high temperature of 1200 °C under nitrogen or argon atmosphere produced a biochar, which was subsequently reacted with D‐tyrosine as a bio‐based dispersion agent under sonication to obtain graphene [75]. The graphene derived from biomass contained many impurities, whereas the graphene synthesized from graphite is usually purer. To avoid contamination, some researchers used pure lignin to produce fine graphene through a stepwise pyrolysis process [76]. The biomass type may affect the properties of graphene as well. Populus wood was mixed with KOH before carbonization under nitrogen. Surprisingly, the graphene‐like carbon material was found on the structure of the obtained biochar [20]. Sucrose, xylitol, and glucose were also used as starting materials for graphene synthesis. The carbonization of sugar mixed with FeCl 3generated graphene‐like carbon materials, which presented excellent catalytic activity in the hydrogenation of nitrobenzene [77]. Even though graphene is one of the beneficial carbon materials, the production of graphene from biomass without any impurities remains a challenge.
To meet the requirement to be a heterogeneous catalyst or support, the physicochemical properties and thermal stability of the material are crucial as these dictate the catalytic efficiency. As previously described, bio‐based carbon materials have predominant characteristics that benefit their application as a direct catalyst or catalyst support. Biomass‐derived porous carbon materials with high porosity provide a greater number of active sites. Moreover, the pore size and specific surface area can be tailored via the process parameters (temperature, time, heating rate, type of biomass, activating agent, etc.). Several forms of bio‐based carbon materials including granules, pellets, tubes, and sheets can be produced. A variety of natural oxygenated surface functional groups in bio‐based carbon materials is useful for metal adsorption when used as a support. These functional groups can also enhance reactant adsorption in heterogeneous catalysis. Some bio‐based carbon materials are cheaper than commercial catalysts and supports, e.g. zeolite, silica, and alumina. Before going into the details on the applications of promising bio‐based carbon materials in a field of catalysis, some of their properties are summarized in Table 2.3.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «High-Performance Materials from Bio-based Feedstocks»
Представляем Вашему вниманию похожие книги на «High-Performance Materials from Bio-based Feedstocks» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «High-Performance Materials from Bio-based Feedstocks» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












