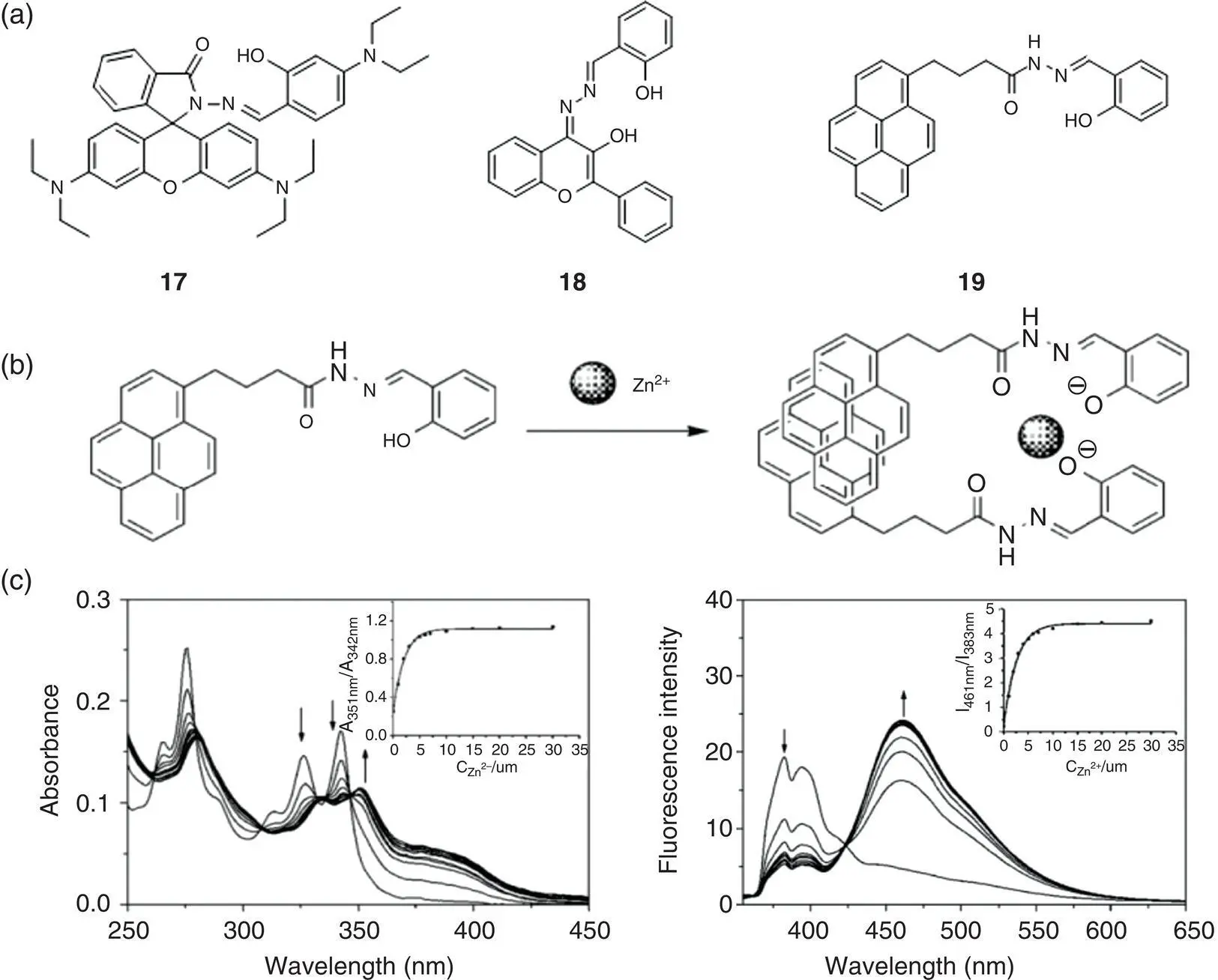
Figure 3.9 (a) Chemical structures of ratiometric fluorescent probes 17– 19based on SSB. (b) The proposed 1 : 2 metal‐to‐ligand ratio binding model of probe 19with Zn 2+. (c) Absorption and ratiometric fluorescence spectrum response of probe 19with Zn 2+.
Source: Reprinted from Ref. [27] (Copyright 2009 Elsevier B.V.).
3.2.2 Biologically and Environmentally Related Molecular Detection and Imaging
Except large amounts of reports for metal ion detection, SSB derivatives were also designed for inorganic species, small organic molecules, as well as macromolecules in biologically and environmentally related analytes. Many reports concern for charged species detection; the probes are usually designed by ionizing AIEgens to increase their solubility in water and rendering them nonfluorescent or weakly fluorescent. In the presence of target molecules with opposite charges, molecular aggregates formed and fluorescence‐enhanced signal was achieved. A series of fluorescent probes with excellent detection properties have been developed by the functionalization of the salicylaldehyde moieties [30] and were successfully applied to the detection of inorganic species [31–38], environmental pollutants [7, 37], biological inorganic molecules [38, 39], amino acids [40, 41], proteins [42–44], enzymes [45–47], and so forth. Herein this section is divided into three aspects: (i) inorganic species, (ii) small biomolecules, and (iii) biomacromolecules to introduce the design and applications for SSB‐based probes in chemical and biological sensing.

Figure 3.10 (a) Chemical structure of probe 17and binding mode with zinc ion verified by 1H‐NMR and mass spectra. (b) Fluorescence intensity ratio ( F 530/ F 475) of 17(10 μM) and 17with Zn 2+(10 μM) in the absence and presence of different metal ions. (c) Absorption spectra of 17(10 μM) in the presence of 0–50 μM Zn 2+in 90% (v/v) ethanol/water (10 mM (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) (HEPES), pH 7.0). Inset: Job plot analysis of 17and Zn 2+at a total concentration of 33 μM, indicating the formation of a 1 : 1 metal‐to‐ligand complex. (d) Fluorescence excitation (a) and emission (b) spectra of 17(10 μM) in the presence of different concentrations of Zn 2+in 90% (v/v) ethanol/water (10 mM HEPES, pH 7.0). Inset: fluorescence intensity at 530 and 475 nm and the ratio of F 530: F 475as a function of Zn 2+concentration added to 17.
Source: Reprinted from Ref. [28] (Copyright 2010 John Wiley & Sons, Ltd.).
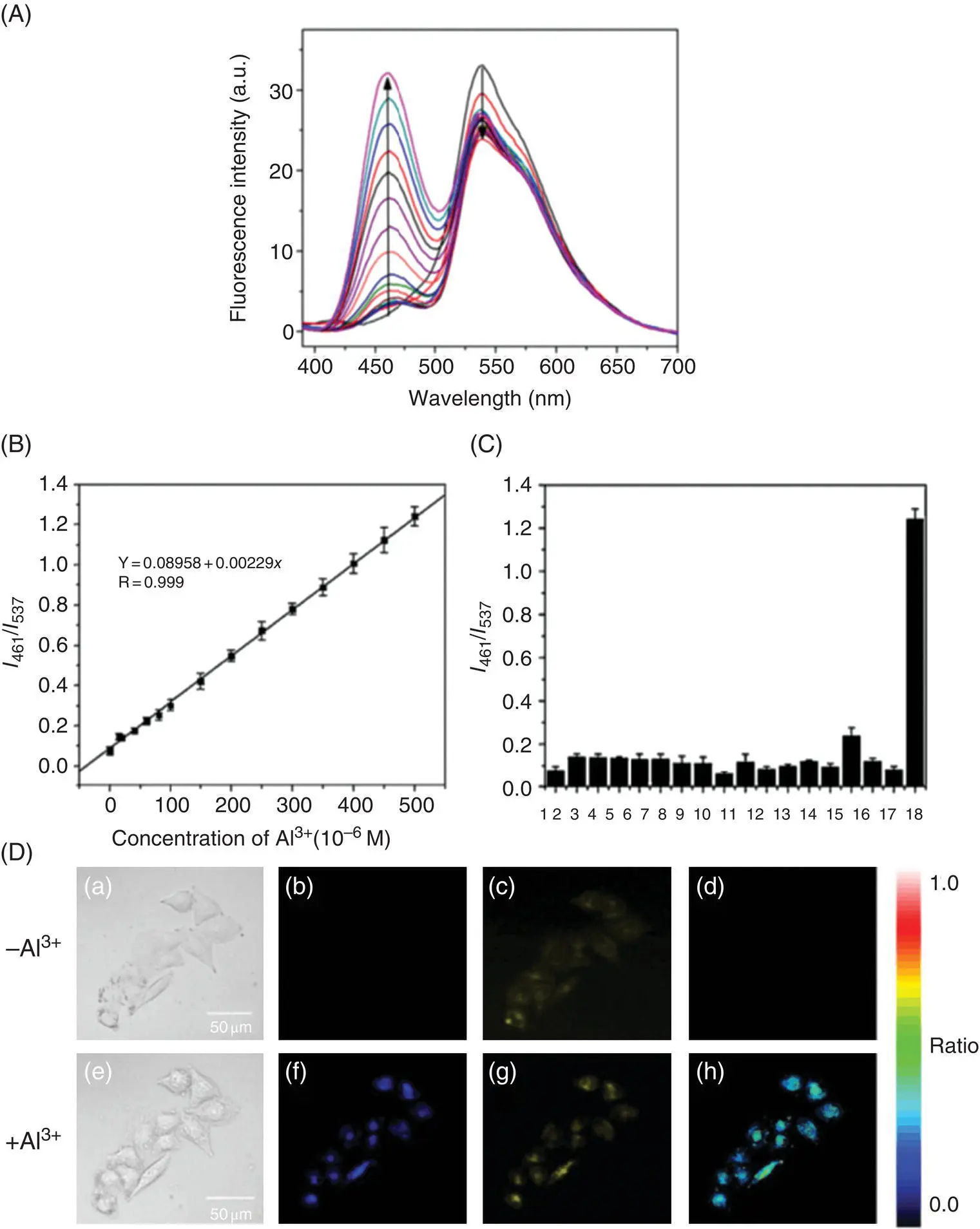
Figure 3.11 (A) Fluorescence spectra of 18(20 mM) upon the addition of different concentrations of Al 3+(0–500 mM) in 10 mM HEPES buffer at pH 7.0 (containing 0.2% DMSO). Excitation wavelength is set at 370 nm. (B) Calibration curve based on the ratio of fluorescence intensities ( I 461/ I 537) as a function of Al 3+concentrations; error bar represents three repeated experiments. (C) Fluorescence intensity ratio ( I 461/ I 537) of 18(20 mM) upon the addition of 500 mM metal ions in 10 mM HEPES buffer at pH 7.0 (containing 0.2% DMSO). Ions from 1 to 18: blank, Li +, Na +, K +, Ca 2+, Mg 2+, Ba 2+, Sr 2+, Fe 2+, Fe 3+, Co 2+, Cu 2+, Hg 2+, Ag +, Cd 2+, Mn 2+, Ga 3+, and Al 3+. (D) Confocal fluorescence images of live HeLa cells. (a–d) The cells were incubated with 18(5.0 mM) for 30 minutes; (e–h) the above cells upon the addition of 200 mM Al 3+were then incubated for another 20 minutes; (a) and (e) bright‐field transmission images; (b) and (f) blue channel images at 429–469 nm; (c) and (g) yellow channel images at 511–611 nm; (d) and (h) ratio images generated from (b) and (c) and (f) and (g), respectively. The excitation was set at 405 nm.
Source: Reprinted from Ref. [26] (Copyright 2014 Elsevier B.V.).
The detection of inorganic species such as CN −, F −, UO 2 2+, S 2−, and ClO −is of great significance in environment, biology, and industry. Cyanide (CN −) is one of the most powerful poisons. It affects vascular, vision, cardiac, endocrine, and metabolic functions and causes fatal damage to the nervous system. The fluoride ion (F −) is very useful in the treatment for osteoporosis and orthodontics. However, excess F −leads to fluorosis, which results in the increment in the bone density. Taking the advantage of hydrogen bonding with the hydroxyl group of SSB, the detection of such basicity anions like CN −and F −is facile by SSB fluorescent probes with high sensitivity and satisfactory selectivity. As shown in Figure 3.12a and b, after adding CN −, probe 20undergoes deprotonation due to the basicity of CN −. Cyclization reaction occurred under the catalysis of CN −, and the corresponding benzoxazole formed gradually in this process, therefore lighting up the fluorescence [34]. The detection limit is 5.92 × 10 −7M, lower than the WHO guideline of CN −in drinking water (1.9 μM). The competitive experiments reveal high sensing selectivity and sensitivity of 20for CN −over other anions ( Figure 3.12c). Test papers were also prepared for the practical application of cyanide detection. By influencing hydrogen bonding, SSB‐based fluorine ion fluorescent probes were also reported. Figure 3.12d lists some SSB probes for F −detection. The possible processes and mechanisms are proposed, as demonstrated in Figure 3.12e. The addition of F −resulted in a blue‐shifted emission, which was increasing gradually with an increasing concentration of F −. This change was explained by the disruption of the existing six‐membered hydrogen bonding, involving the hydroxyl group and imine nitrogen, which allow the ESIPT process through deprotonation of the phenolic hydroxyl group. Excellent experimental results of detection limits and selectivity over other anions were also obtained in real sample detection ( Figure 3.12f, g).
Chen et al. reported a fluorescence turn‐on SSB probe 25for uranyl ion (UO 2 2+) detection with high efficiency [32]. As one of the radioactive metal elements, uranium is an important raw material for the nuclear industry. At present, uranium‐based nuclear power facilities have gradually increased their proportion in power generation facilities in various countries. Metal uranium is extremely radioactive and chemically toxic, with a half‐life of hundreds of millions of years, which can cause lasting disturbances and damage to the immune, reproduction, and hematopoietic systems of the organism. Uranyl ion (UO 2 2+) is the most commonly existing formation of uranium in natural water; therefore, the detection of UO 2 2+in water is of great significance for the assessment of water pollution. As illustrated in Figure 3.13a, 25undergoes a complex interaction and forms aggregates with the addition of UO 2 2+, exhibiting a fluorescence enhancement at 540 nm, which was linearly related to the concentration of UO 2 2+in the range of 1–25 ppb. The limit of detection was achieved as low as 0.2 ppb with a relative standard deviation (RSD) of 1.3% ( Figure 3.13b, c). Such a detection method was successfully utilized in quantifying UO 2 2+in fuel processing wastewaters. Other fluorescent probes based on salicylaldehyde azine derivatives for the detection of sulfur(38) (S 2−), hypochlorite(37) (ClO −), and so on were also developed by the modification of a metal complex of SSB.
Читать дальше















