The detection range of common metal ion fluorescent probes is generally at the nanomolar and micromolar levels. Few fluorescent probes detect metal ions at the millimolar level. This is because when the metal ion concentration is too high, an aggregation‐caused quenching (ACQ) effect may occur and thus result in an emitting annihilation of classical fluorescent probes. However, the Ca 2+concentration related to human diseases is generally in the millimolar range, which begets great challenges for the development of practically applied fluorescent probes for measuring the Ca 2+concentration in the human body. Tang's group has developed a calcium ion fluorescent probe 16with a detection range of 0.6–3.0 mM, which is suitable for the detection of abnormally high blood calcium concentration ( Figure 3.8A) [17]. The fluorescence quantum yield of the probe in the pure tetrahydrofuran (THF) solution was 0.23%, and it increased to 10.6% in the solid state. With the increase of Ca 2+concentration, the emission of probe 16at 560 nm was significantly enhanced ( Figure 3.8B). Some common metal ions and biological molecules (such as bovine serum albumin [BSA], porcine hemoglobin [PHB], and fetal bovine serum [FBS]) exhibit no interference with the fluorescence spectrum of the probe ( Figure 3.8C). UV–vis absorption and high‐resolution mass spectrometry (HRMS) and isothermal titration calorimetry (ITC) determined that the coordination number between probe 16and calcium ions was 1 : 1. Transmission electron microscopy (TEM) showed that probe 16formed fibrous aggregates in the presence of calcium ions and irregular aggregates in the absence of calcium ions. The formation of regular aggregates induced by Ca 2+significantly enhanced fluorescence. Biological imaging of calcium deposits was performed in the soft tissue of human squamous meningioma and the cracks on the surface of the bovine bone to test the practical applicability of probe 16, and the results were satisfactory ( Figure 3.8D, E). Compared with the commonly used calcium ion probe calcein, probe 16shows advantages of a weak background signal, wash‐free imaging, and higher sensitivity.
Most metal ion probes are tested based on the fluorescence “on–off” mechanism, and the detection result is only related to the fluorescence intensity at a single wavelength, while the ratiometric fluorescence probe takes the advantage of the ratio change of the fluorescence intensity at two wavelengths for analyte detection, which can minimize errors caused by differences in experimental conditions and self‐emission of samples. Figure 3.9shows a summary of ratiometric metal ion fluorescent probe structures based on SSB. Ratiometric fluorescent probes generally contain a stable fluorescent emission moiety and an active site that can react with metal ions. After reacting with metal ions, a fluorescent emission at another wavelength is generated, and the original emission peak intensity is unchanged or decreased, thereby realizing ratiometric detection [26–29].
Tong et al. developed a dual‐wavelength ratio fluorescent probe 17for Zn 2+based on rhodamine B salicylaldehyde fluorene derivative [28]. In general, salicylaldehyde hydrazone structure can generate a strong fluorescence enhancement response with zinc ions, but the fluorescence intensity is low in the absence of metal ions. In order to achieve the effect of the dual‐wavelength ratiometric response, the probe needs to have a similar quantum yield before and after the reaction with the metal ions. Rhodamine dyes have a longer excitation wavelength, higher quantum yield, and suitable water solubility. Combining 4‐ N,N ‐diethylamine salicylaldehyde hydrazone structure with rhodamine dyes, ratiometric zinc ion probe 17was designed and synthesized. By exciting the fluorescence at the iso-absorption point at 400 nm, the maximum emission wavelength is red-shifted by 17nm before and after the probe 17binding with zinc ions, and the iso-emission point appears at 500 nm ( Figure 3.10b, c). Under the UV lamp, a clear fluorescent color change of the solution from dark blue-green to yellow-green was achieved and observed by naked eye. The fluorescence intensity ratio at 530 and 475 nm is linear in the Zn 2+concentration range of 0–10 μM and is independent of other interfering ions. The minimum detection limit is 0.05 μM. Other probes containing rhodamine and salicylaldehyde hydrazone for Zn 2+detection were also synthesized, and the salicylaldehyde structure is found to be indispensable for ratiometric detection of zinc ions due to the different emissions of the probe and probe–zinc complex, as shown in Figure 3.10a.
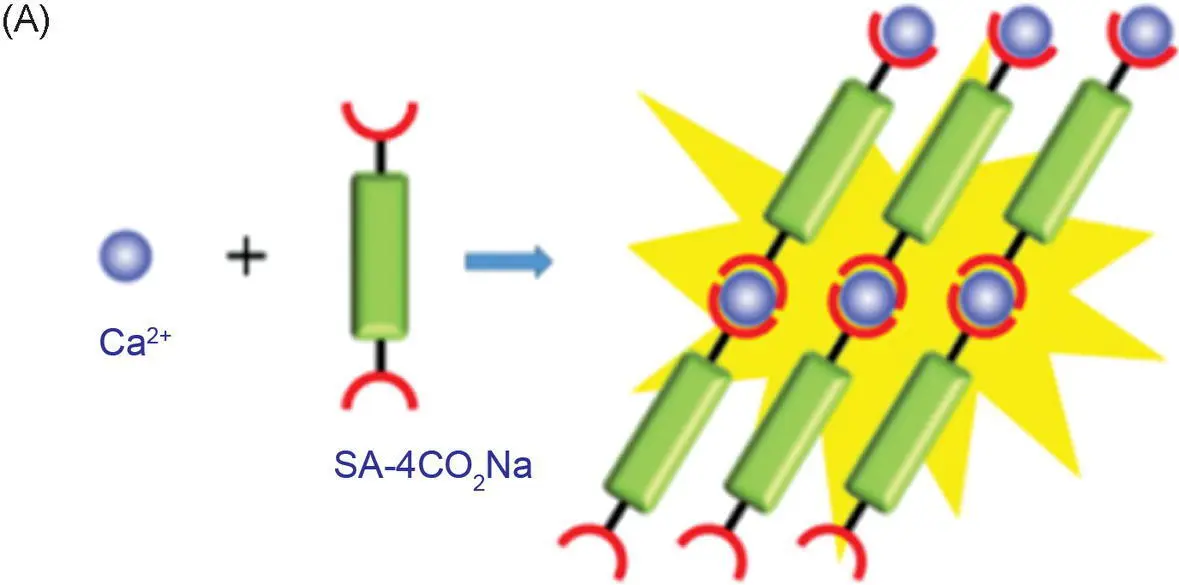
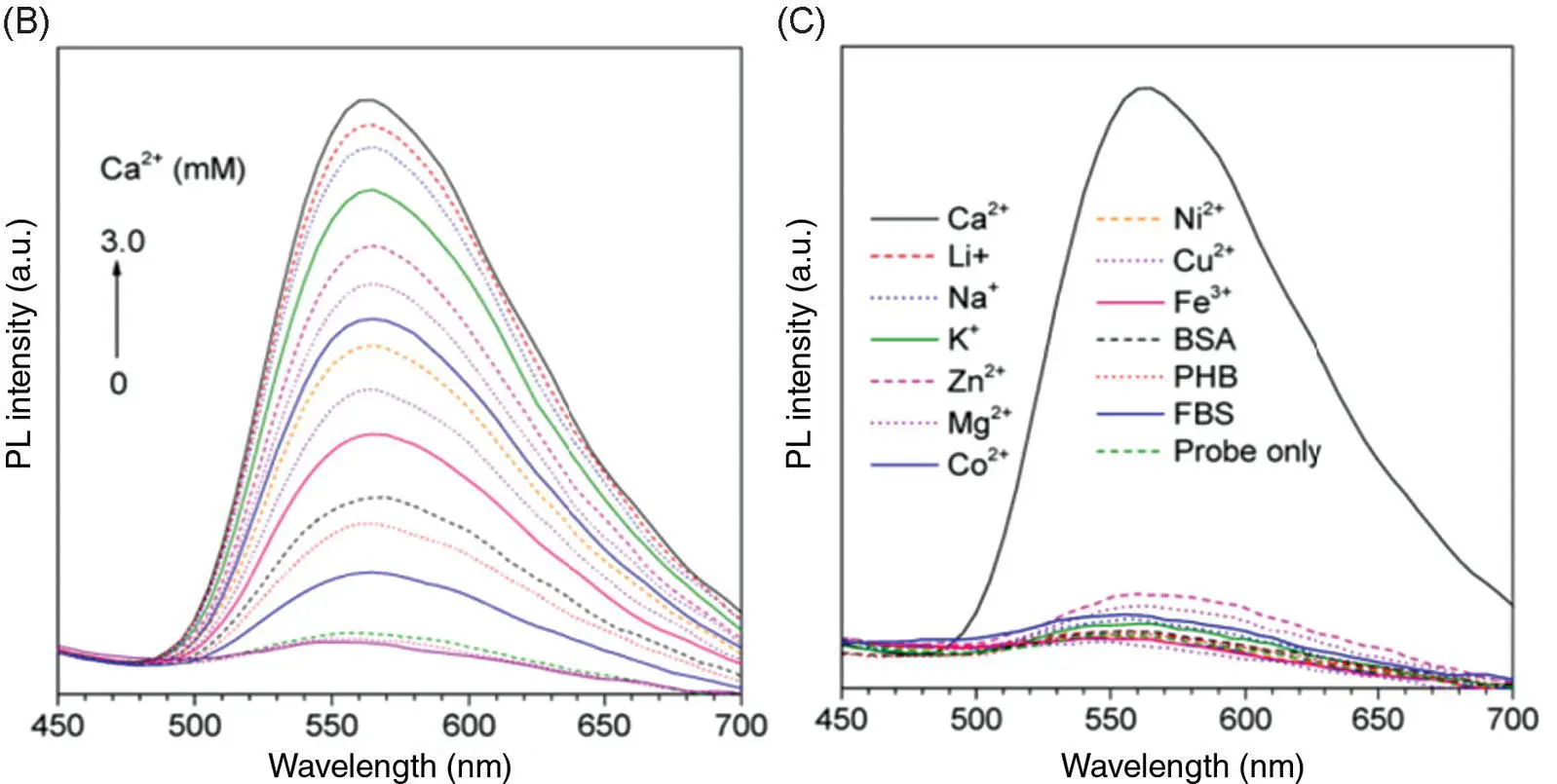
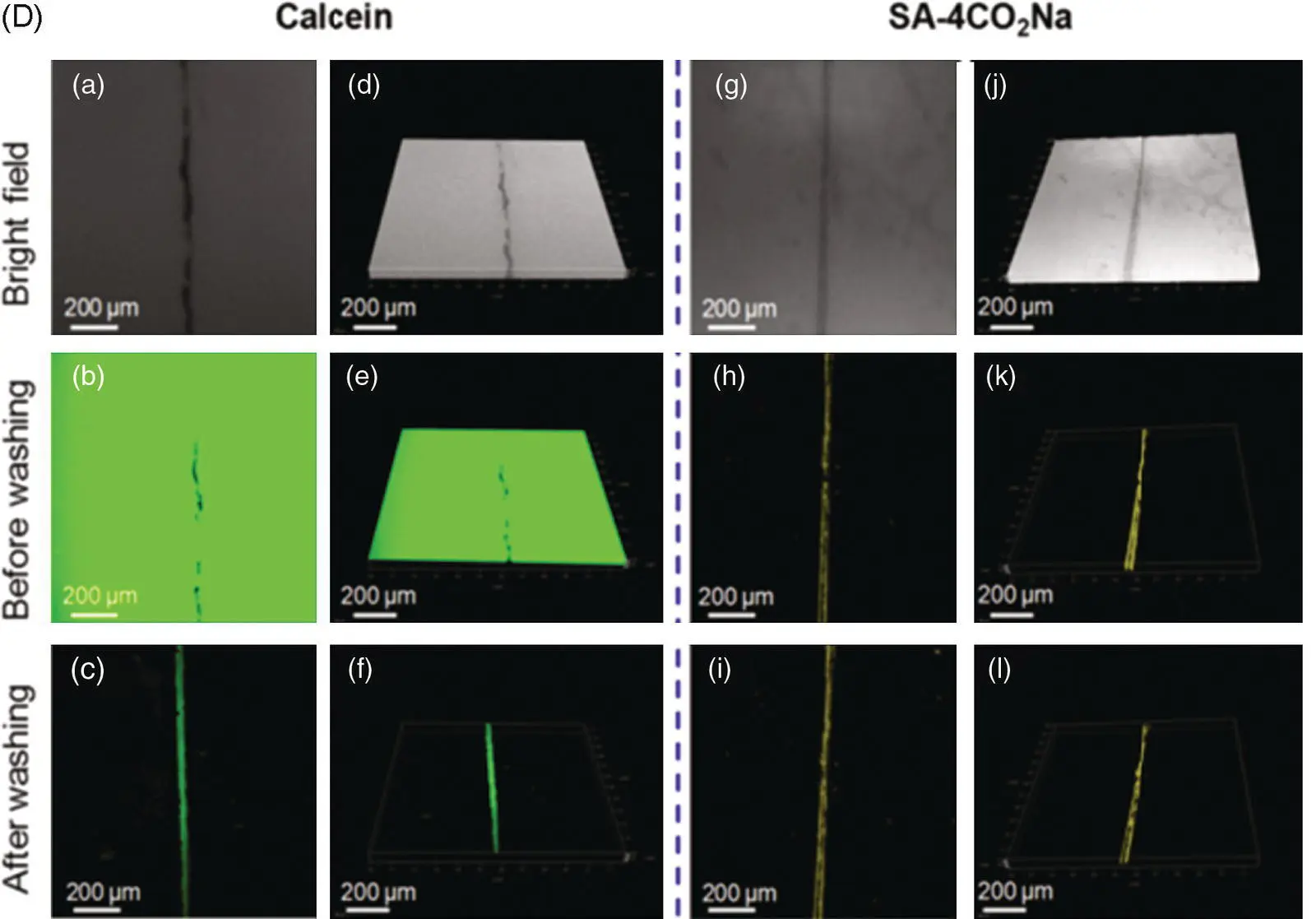
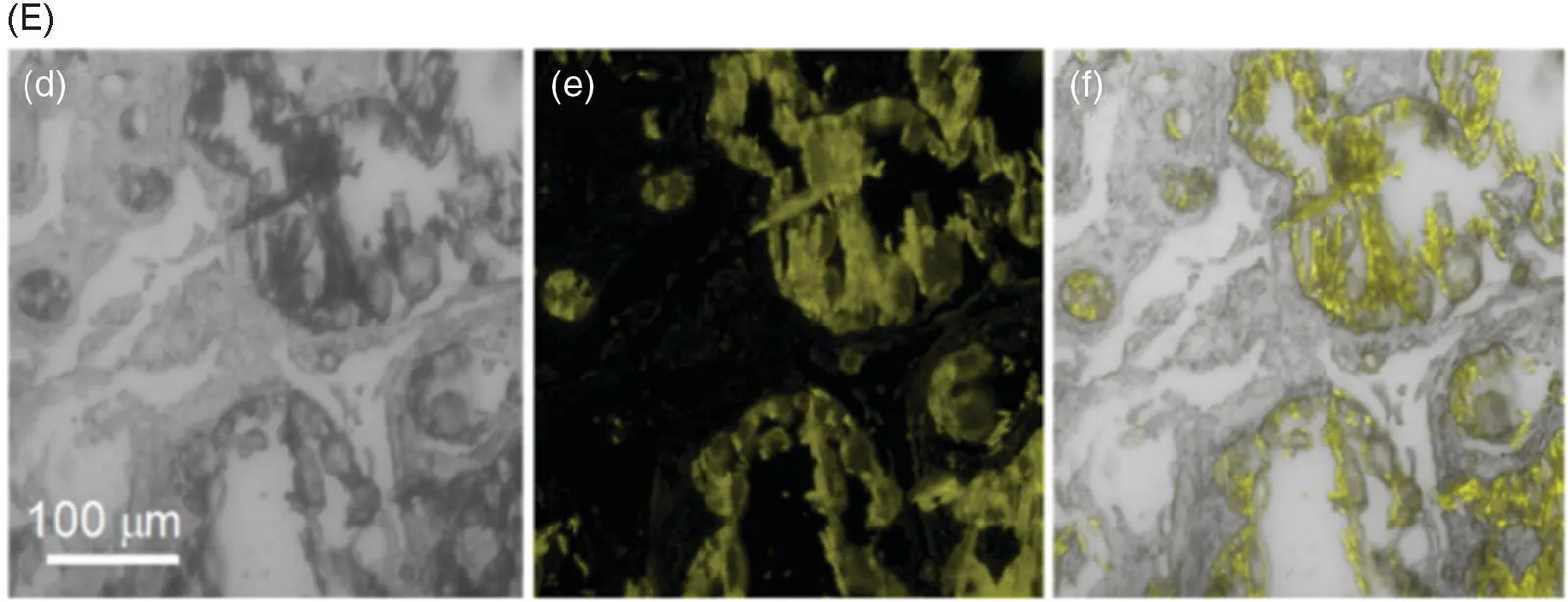
Figure 3.8 (A) Illustration of the light‐up detection of Ca 2+with probe 16. (B) Photoluminescence (PL) spectra of 16treated with CaCl 2at different concentrations in phosphate‐buffered saline (PBS) buffer solution (pH = 7.4). (C) PL spectra of 16in PBS buffer upon the addition of various metal ions and biological molecules. (D) Confocal laser scanning microscopy (CLSM) images of bovine bone microcracks by staining with calcein and 16: (a–c) whole‐projection images (z stack) and (d–f) 3D images of the microcrack stained with calcein; (g–i) whole‐projection images (z stack) and (j–l) 3D images of the microcrack stained with 16. (E) CLSM images of CaCl 2embedded in calcium deposits in psammomatous meningioma slice: (d) the bright‐field images; (e) the fluorescence images; and (f) the merged images.
Source: Reprinted from Ref. [17] (Copyright 2018 American Chemical Society).
A ratiometric fluorescent probe 18using 3‐hydroxyflavones and salicylaldehyde hydrazone Al 3+ion was reported [26]. The fluorescence color of the probe 18solution changed from yellow to white after the addition of aluminum ions due to the emission intensity at 461 nm being increased but the intensity at 537 nm decreased as shown in Figure 3.11A. I 461/ I 537has a linear relationship with the concentration of aluminum ions ( Figure 3.11B). The lowest detection limit measured under the experimental conditions is 0.29 μM (7.8 ppb), and the fluorescence response is stable in the pH range of 3.0–10.0. DLS showed that the average diameter of aggregates decreased significantly before and after the interaction with aluminum ions, indicating that aluminum ions and probe 18could form a water‐soluble complex, but there were still aggregates of probe 18in the solution, thus showing that a white color fluorescence originated from the mixed color of the probe aggregate and the complex. The coordination ratio between probe 18and the aluminum ion is 1 : 1 based on Job's plot; NMR titration indicates that both hydroxyl groups in the probe molecule participate in the coordination with the aluminum ion. Selectivity experiments with other metal ions show that a significant increase in I 461/ I 537was only found in the presence of Al 3+( Figure 3.11C). HeLa cells were selected for intracellular experiments, and the fluorescence signal was only observed in the yellow light channel. With the addition of aluminum ions, the yellow light signal was basically unchanged under the confocal microscope, and the fluorescence of the blue light channel gradually increased, showing that probe 18can successfully perform ratio imaging of aluminum ion in cells ( Figure 3.11D).
Читать дальше
















