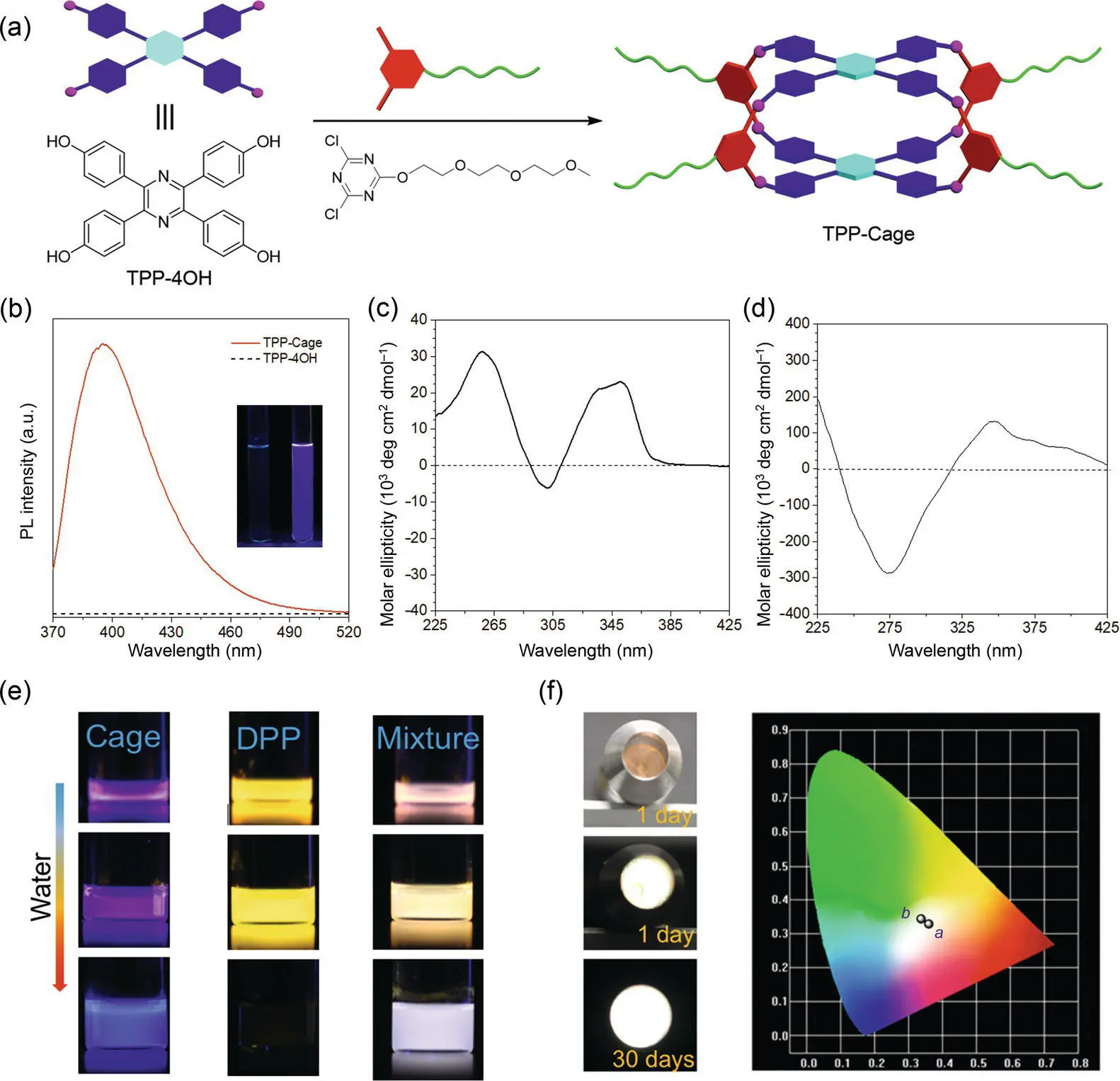
Figure 1.3 TPP‐based chiral cage for self‐assembly to achieve white‐light emission. (a) Synthetic route to the TPP cage. (b) PL spectra of TPP‐4OH and TPP cage in THF; their concentration is 50 μM. Inset: their photographs taken under a UV light irradiation. CD spectra of the TPP cage in (c) acetonitrile and (d) acetonitrile/water mixture with a 95% water content; the concentration is 100 μM. (e) Photographs of the TPP cage, DPP, and complex in THF and THF/water taken under a UV light irradiation. (f) Photographs of the complex‐doped PEG film‐casted flashlight during different times and the CIE coordinate of its emission spectra.
Obviously, the TPP cage has a hydrophobic cavity and is potential to capture the guest molecules. Thus, the investigation of its host–guest chemistry is expected. Diketopyrrolopyrrole (DPP) is an ACQ chromophore with a yellow emission in the solution. However, the size of DPP can fit the cavity volume of the TPP cage properly and the supramolecular assembly complex between them can be obtained readily. The successful formation of the complex is proved by the NMR titration and analysis, and the UV–vis spectra indicate a complex ratio of 1 : 1. As DPP has an ACQ effect, its emission is easy to be quenched once aggregated. However, DPP shows a strong yellow emission in the cage even when the complex is in the aggregate state ( Figure 1.3e). It demonstrates that the DPP molecules have been isolated by the TPP cage while the π – π stacking has been prohibited to overcome the ACQ effect, therefore providing a flexible strategy to design highly efficient solid‐state luminescent materials with ACQ luminophores. On the other hand, the strong blue emission of the TPP cage sustains. The combined emissions of blue and yellow lights can be observed in the assembly. The emissions of the complex change somewhat from the solution state to the aggregate state. For example, it shows a pink emission in THF. Upon addition of water, the spectrum shifts to white light, which is probably to the subtle variation in emissions of the TPP unit and DPP by changing the microenvironment. Finally, Tang also demonstrates its potential application as white‐light emitters. By doping the complex in the PEG with a weight ratio of 1%, a white‐light‐emissive PEG film can be formed. Further casting the film onto the UV flashlight gives a stable white‐light source for illumination ( Figure 1.3f).
1.3.4 Metal–organic Framework
MOFs are a kind of organic–inorganic crystals that consist of metal ions and organic molecules as center and ligand, respectively. The two‐ or three‐dimensional rigid framework makes them porous with a large specific surface area and tunable pore size, thus very promising for utilization as porous materials [58]. MOFs can also be developed as luminescent materials because of their potential luminescent properties of metal ions and ligands. Recently, some luminescent MOFs with TPE‐based units as ligand have been designed to exhibit sensing and photocatalytic behaviors [59, 60]. As TPP shows the similar structural symmetry to TPE, TPP‐based MOFs are thus desired to be developed.
Yin developed MOFs with Ln 3+ions, including Eu 3+, Tb 3+, and Gd 3+as centers and tetracarboxyl‐substituted TPP (TPP‐4COOH) as ligand [61]. The coordination process can proceed in the mixed solution and be monitored by turn‐on emission, indicative of a coordination‐induced emission. However, different ion centers may cause different optical properties of MOFs ( Figure 1.4a). Eu 3+‐MOF possesses obvious dual emissions at around 420 and 630 nm, which are originated from the TPP unit and Eu 3+, while only the emission of TPP can be observed in Gd 3+‐MOF. The emission of Tb 3+in Tb 3+‐MOF in the long‐wavelength region is weak but still can be discerned. This is due to the different energy gaps between the singlet state and triplet state of a ligand and the triplet state of a ligand and the excited state of metal ions, which determines the different intersystem crossing and sensitization efficiencies ( Figure 1.4a). In other words, the emission of metal ions is influenced by the combined consideration of the orbital properties of both metal centers and organic ligands.
Although the various metal ions have been employed, their MOFs show similar structural morphologies as revealed by PXRD analysis, indicative of the university of choosing TPP to design MOFs. Moreover, the emission of the TPP unit in MOFs is somewhat blue‐shifted than those of TPP‐4COOH. As the formation of MOF can be regarded as a crystallization process, TPP must adjust the conformation to fit the crystal lattice, while a much twisted molecular structure will shorten the conjugation to blue‐shift the emission, which is in accordance with the results obtained by the single‐crystal diffraction analysis ( Figure 1.4b). On the other hand, the Φ Fof TPP‐4COOH was measured to be 12.9% in the solid state. However, after being introduced into MOF, the Eu 3+‐MOF shows dual emissions with a remarkably increased Φ Fof 60.8%. It demonstrates a useful strategy to construct highly efficient luminescent materials by utilizing coordination‐induced emission and antenna effect.

Figure 1.4 Functional MOFs with TPP‐4COOH as ligand for sensing. (a) Intersystem crossing and sensitization effect determined by the energy level properties of ligands and metal ions. (b) Crystal structure of Eu 3+‐MOF. (c) PL spectra of Eu 3+‐MOF upon the addition of arginine with different concentrations. (d) Model of hydrogen formation between MOF and arginine.
As two fluorescent channels are observed in the Eu 3+‐MOF, it is potential to develop a ratiometric fluorescent probe based on it. Interestingly, Eu 3+‐MOF has a fluorescent response to arginine, which is an essential amino acid in the body associated with a variety of diseases like cardiovascular and cerebrovascular diseases and hyperammonemia, etc [61]. Upon the addition of arginine, the emission of the TPP unit enhances gradually, while those of Eu 3+changes less, thus making the signals ratiometrically detectable ( Figure 1.4c). It is due to the nitrogen atoms in the central pyrazine ring of the TPP unit, which provides sites for binding. On the other hand, the pores in the MOFs are competent in accommodating the arginine. The arginine, therefore, can enter into the MOF and form a hydrogen bond with TPP, thus offering an additional steric hindrance to restrict molecular motions to enhance emission ( Figure 1.4d). The emission behavior of Eu 3+is less influenced because no interaction is found between Eu 3+and arginine. Besides, by replacing the TPP‐4COOH ligand with tetracarboxyl‐substituted tetraphenylbenzene, the resulting MOF does not possess a response to arginine, further proving the importance of the introduction of an AIE‐active TPP unit. The sensor also shows a good selectivity against other amino acids and high sensitivity with a detection limit as low as 15 nM.
The study of TPP dated back to the mid‐nineteenth century, while its real structure was established by Japp and Wilson in 1886. Since that, more studies are focused on synthetic methodologies and medicinal chemistry. It is until 2015 that Tang found TPP to be AIE‐active and have merits of easy synthesis, facile medication, good stability, and tunable electronic property, etc. This, therefore, evolves from a variety of functionalities based on tetraphenylpyrazines and makes its researches very attractive.
Читать дальше














