1 ...7 8 9 11 12 13 ...42
1.2 Synthesis of TPP‐based AIEgens
1.2.1 Cyclization Reaction
Although TPP was found to be AIE‐active by Tang in 2015, its research dates back to the mid‐nineteenth century. The early synthesis of TPP was carried out by Laurent in 1845 and later by Erdmann in 1865 [40, 41]. The reaction was carried out by heating benzoin with ammonium chloride at 100 °C for four to six hours. Three main products formed after the reaction named as benzoinam (formula C 28H 24N 2O), benzoinimide (formula C 14H 11N), and lophine, respectively. In 1886, Japp and Wilson reinvestigated the reaction. They found that a yellow crystalline powder of benzoinimide formerly obtained was actually impure. After recrystallization with a large amount of alcohol, colorless, slender, and lustrous crystals were collected. Further elemental analysis study indicated that the formula of the compound agreed better with C 28H 20N 2than with C 14H 11N. That is, the name of benzoinimide given by Erdmann is misleading. They thus renamed the compound as ditolane‐azotide and also gave the prototype structure of TPP in the text [42]. It is the first report of TPP.
Since that, the researches on TPP were burgeoning. In 1937, Davidson, Weiss, and Jelling studied the action of ammonia on benzoin [43]. Besides the generated TPP, an imidazole derivative of 2‐methyl‐4,5‐dipenylglyoxaline and tetraphenyldihydropyrazine was formed. It represented the most classical rudiment in the later preparation of TPP. The reaction mechanism was given as below: the carbonyl group of benzoin was possible to convert to imine in the presence of ammonia, followed by generating carbinamine by structural rearrangement, while the hydroxyl group nearby was converted to the carbonyl group. Two formed intermediates can undergo cyclization reaction by condensation and removal of water. After structural tautomerization, a resembling product of tetraphenyldihydropyrazine was formed to oxidize to TPP. On the other hand, the intermediate was also easy to react with the solvent of acetic acid by the amino group and then formed 2‐methyl‐4,5‐dipenylglyoxaline by cyclization ( Scheme 1.1). The formation of tetraphenyldihydropyrazine was evidenced by the orange color of the reaction mixture soon after refluxing of the starting materials. Remarkably, oxygen plays a crucial role in the conversion of tetraphenyldihydropyrazine to TPP. It is proved that the yield of reaction solution bubbled with air is higher than that in the closed environment. Nevertheless, the total yield of TPP is in the range from 53 to 57%, and the tetraphenyldihydropyrazine cannot convert to TPP completely even in the presence of enough oxygen. After filtering out the formed crude TPP precipitates, the unreacted tetraphenyldihydropyrazine in the mother liquor can be further oxidized to TPP by adding nitric acid until the orange color of solution was discharged.
Tang also reported the synthesis of TPP by refluxing benzoin, ammonium acetate, and acetic anhydride in acetic acid for 3.5 hours, while the acetic anhydride acts as a dehydrating agent to remove the water after cyclization ( Scheme 1.2, Route A, Condition 1) [33]. TPP shows the same polarity with the byproducts as monitored by the silica gel plate. The blue emission of TPP disappeared, while only the orange emission was observed on the plate. It is mainly due to energy transfer taking place from TPP to the byproducts, which dramatically quenches its emission. It implies that the purity of TPP after purification must be rather high because the trace of impurities in TPP will obviously affect its photo‐physical properties. TPP and its byproducts show different solubility in acetic acid. TPP is badly dissolved in acetic acid. It precipitates during the reaction, while most of the byproducts were still left in the solution. The crude TPP powder is collected by filtration and shows a yellow appearance due to the presence of trace impurities. However, repeated recrystallization of crude products in a larger amount of heat acetic acid can afford very pure TPP crystals. The yield (34%) is lower than the above studies, probably due to the loss of product during the purification. The convenience in preparation and purification of TPP makes it very promising for further deep investigations.
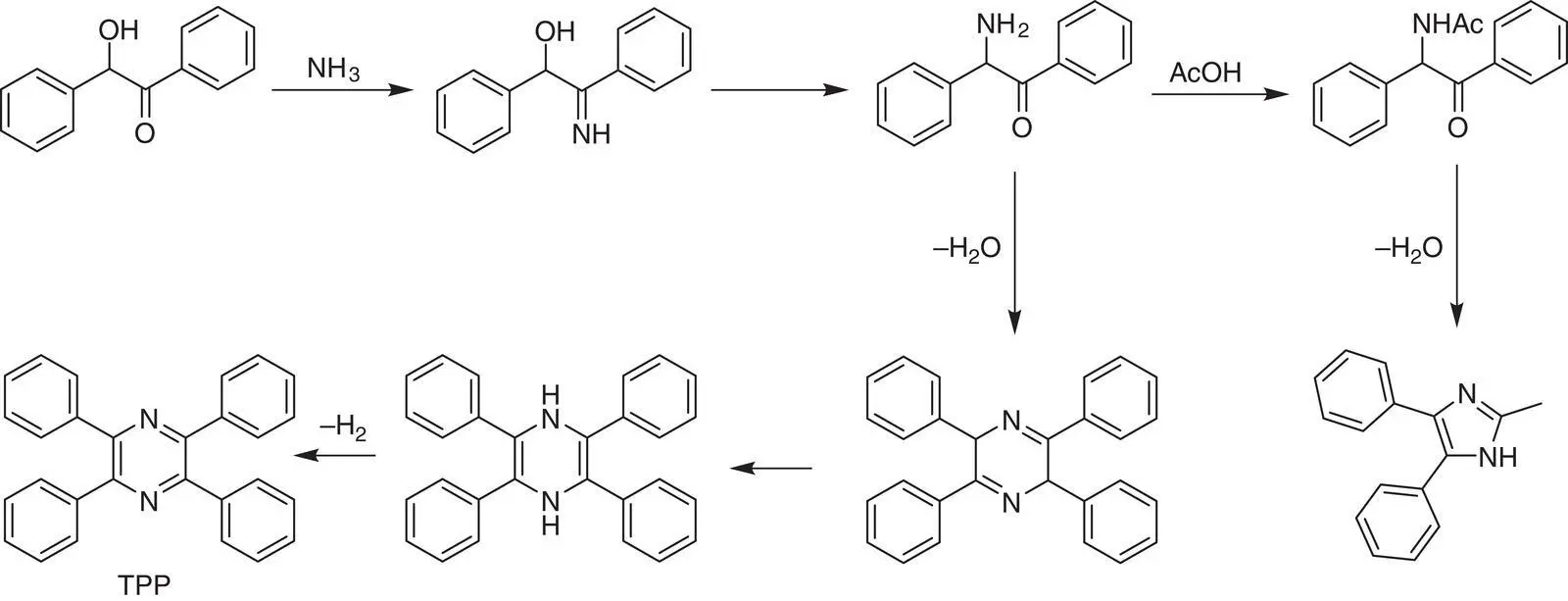
Scheme 1.1 Reaction mechanism of synthesizing TPP with benzoin and ammonia.
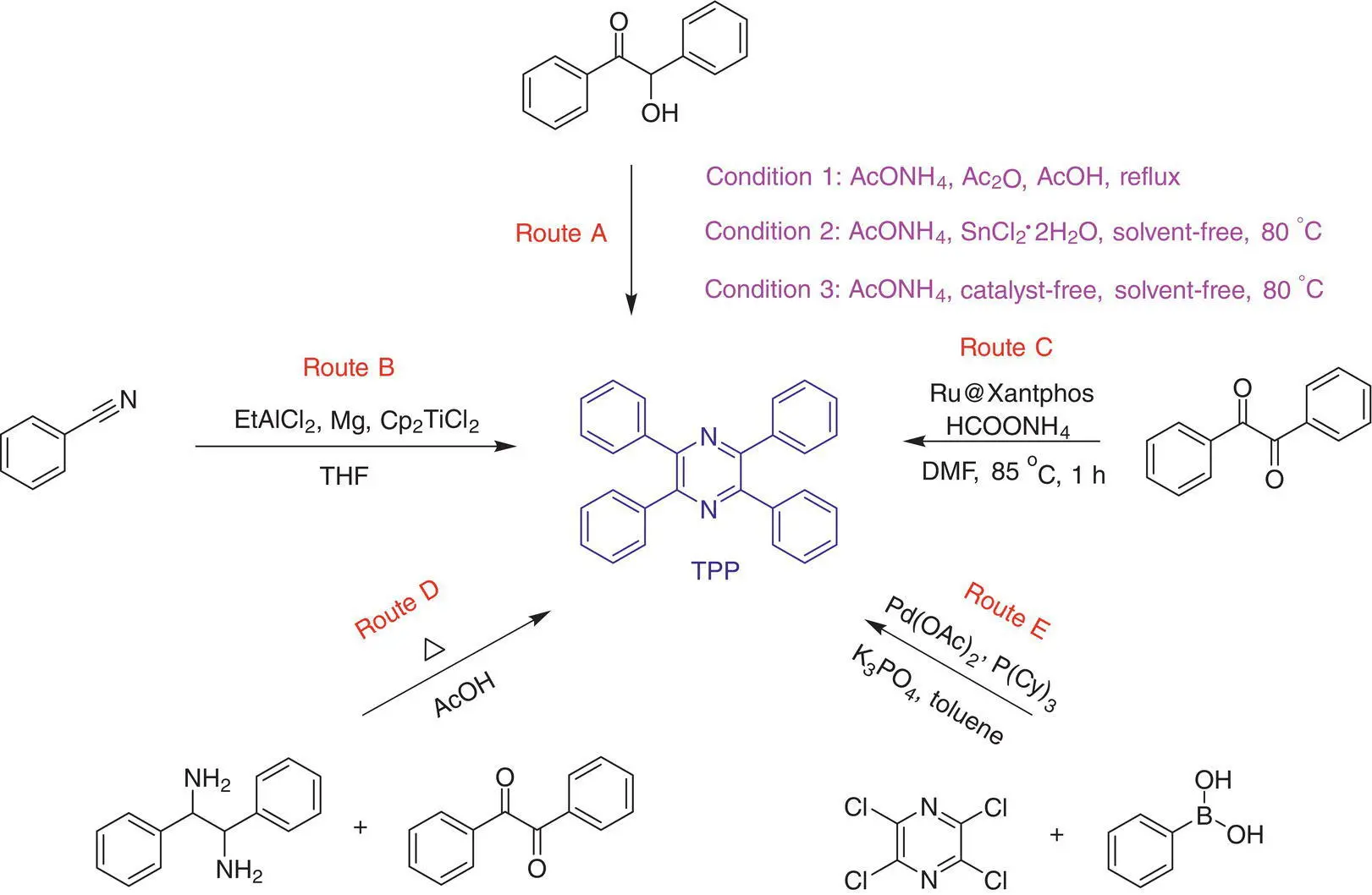
Scheme 1.2 Current synthetic routes to TPP.
This method not only affords TPP readily but is also useful for preparing its derivatives with diverse structures. For example, by using mono‐ or disubstituted benzoin, di‐ or tetrasubstituted TPP derivatives can be easily obtained. The reaction is less affected by the electronic effect of substituents. However, if the substituents are bulky, the crude products are difficult to purify by recrystallization. It is worth noting that two isomers are usually formed by using monosubstituted benzoin in the reaction. Such behavior is similar to that in the preparation of disubstituted TPE derivatives by the McMurry coupling reaction [44]. The presence of isomerization in TPP derivatives is proved by the fact that four resonance signals exist around 148 ppm in their 13C NMR spectra due to the four different chemical environments of carbon atoms in the pyrazine ring [33].
Tamaddon used SnCl 2·2H 2O as a catalyst to synthesize α ‐amino ketones with benzoin and aniline as starting materials under solvent‐free conditions at either 80 °C or microwave irradiation [45]. Although the yields of reaction are high (up to 83%), the undesired byproduct of 1,4‐dibenzyl‐2,3,5,6‐tetraphenyl‐1,4‐dihydropyrazine is formed as examined by the NMR analysis, which is possibly due to the self‐condensation of the in situ formed α ‐amino ketones. Further investigation indicated that under the same catalytic condition, a [2 + 1 + 1 + 2] four‐component reaction of benzoin and ammonium acetate can generate TPP in a high yield of 90% ( Scheme 1.2, Route A, Condition 2). It provides a convenient strategy to prepare tetrasubstituted tetraphenylpyrazines in high yields. The substituents on benzoin are widely alternative. For example, by using methoxyl‐, methyl‐, bromine‐, chlorine‐, and fluorine‐substituted benzoins, the reactions can proceed smoothly. However, as the electron‐donating property of substituents increases, the yield of reactions slightly decreases.
Subsequently, Tamaddon found that the multicomponent reaction can be carried out without SnCl 2·2H 2O as catalyst ( Scheme 1.2, Route A, Condition 3) [46]. That is, SnCl 2·2H 2O is not necessary in the reactions. By directly heating the solid mixture of benzoin and ammonium acetate at 80 °C, the product can be obtained in a very high yield. Overall, this reaction is very close to the original one reported by Laurent, Erdmann, Japp, and Wilson, except that the ammonium chloride has been replaced by ammonium acetate. This optimal reaction condition was confirmed by the author after evaluation of the influence of ammonium salt, solvent, and reaction temperature on the reaction efficiency. The yields remain high in the reaction of benzoin derivatives regardless of the electron effect of the substituents. The reaction mechanism was similar to the previous one. Ammonium acetate releases the ammonia by heating to react with benzoin to generate an intermediate. Because the ammonium acetate is subtly excess, few acetic acid is formed, which can hardly react with the intermediate to produce 2‐methyl‐4,5‐dipenylglyoxaline. On the other hand, the solid‐state reaction allows the full contact of reactant with air. Both the factors may decide a high yield of reaction.
Читать дальше














