1.16 Structures Built of Space‐Filling Polyhedra
This approach to describing crystal structures emphasises the coordination number of cations. Structures are regarded as built of polyhedra, formed by the cations with their immediate anionic neighbours, which link by sharing corners, edges or faces. For example, in NaCl each Na has six Cl nearest neighbours arranged octahedrally; this is represented as an octahedron with Cl at the corners and Na at the centre. A 3D overview of the structure is obtained by looking at how neighbouring octahedra link to each other. In NaCl, each octahedron edge is shared between two octahedra (Fig. 1.31, see later), resulting in an infinite framework of edge‐sharing octahedra. In perovskite, SrTiO 3, TiO 6octahedra link by corner‐sharing to form a 3D framework (Fig. 1.41, see later). These are just two examples; there are very many others, involving mainly tetrahedra and octahedra, leading to a wide diversity of structures. Some are listed in Table 1.5.
Table 1.5 Some structures built of space‐filling polyhedra
| Structure |
Example |
| Octahedra only |
| 12 edges shared |
NaCl |
| 6 corners shared |
ReO 3 |
| 3 edges shared |
CrCl 3, BiI 3 |
| 2 edges and 6 corners shared |
TiO 2 |
| 4 corners shared |
KAlF 4 |
| Tetrahedra only |
| 4 corners shared (between 4 tetrahedra) |
ZnS |
| 4 corners shared (between 2 tetrahedra) |
SiO 2 |
| 1 corner shared (between 2 tetrahedra) |
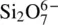 |
| 2 corners shared (between 2 tetrahedra) |
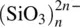 , chains, or rings , chains, or rings |
Although it is legitimate to estimate the efficiency of packing spheres in cp structures, we cannot do this for space‐filling polyhedra since the anions, usually the largest ions in the structure, are represented by points at the corners of the polyhedra. In spite of this obvious misrepresentation, the space‐filling polyhedron approach shows the topology or connectivity of a framework structure and indicates clearly the location of empty interstitial sites.
A complete scheme for classifying polyhedral structures has been developed by Wells and others. The initial problem is a geometric one: what types of network built of linked polyhedra are possible? The variables are as follows. Polyhedra may be tetrahedra, octahedra, trigonal prisms, etc. Polyhedra may share some or all of their corners, edges and faces with adjacent polyhedra, which may or may not be of the same type. Corners and edges may be common to two or more polyhedra (obviously only two polyhedra can share a common face). An enormous number of structures are feasible, at least theoretically, and it is an interesting exercise to categorise real structures on this basis.
The topological approach to arranging polyhedra in various ways takes no account of the bonding forces between atoms or ions. Such information must come from elsewhere. Also, the description of structures in terms of polyhedra does not necessarily imply that such entities exist as separate species. Thus, in NaCl, the bonding is mainly ionic, and physically distinct NaCl 6octahedra do not occur. Similarly, SiC has a covalent network structure and separate SiC 4tetrahedral entities do not exist. Polyhedra do have a separate existence in structures of (a) molecular materials, e.g. Al 2Br 6contains pairs of edge‐sharing tetrahedra, and (b) compounds that contain complex ions, e.g. silicate structures built of SiO 4tetrahedra which form complex anions ranging in size from isolated monomers to infinite chains, sheets and 3D frameworks.
In considering the polyhedral linkages that are likely to occur, Pauling's third rule for the structures of complex ionic crystals (see Chapter 3for other rules) provides a useful guideline. This states that shared edges and, especially, shared faces decrease the stability of a structure, particularly for cations of high valence and small coordination number, i.e. for small polyhedra, especially tetrahedra, that contain highly charged cations. When polyhedra share edges and, particularly, faces, the cation–cation distances (i.e. the distances between the centres of the polyhedra) decrease and the cations repel each other electrostatically. In Fig. 1.28 are shown pairs of (a) corner‐sharing and (b) edge‐sharing octahedra. The cation–cation distance is clearly less in the latter and for face‐sharing octahedra, not shown, the cations are even closer. Comparing edge‐shared octahedra and edge‐shared tetrahedra (b, c), the cation–cation distance M–M (relative to the cation–anion distance M–X) is less between the tetrahedra because the MXM bridging angle is 71° for them and 90° for the octahedra. A similar effect occurs for face‐sharing tetrahedra and octahedra. In Table 1.6, the M–M distances for various polyhedral linkages are given as a function of the M–X distance. The M–M distance is greatest for corner‐sharing tetrahedra or octahedra and is least for face‐sharing tetrahedra. For the sharing of corners and edges, the M–M distances given are the maximum possible. Reduced distances occur if the polyhedra are rotated about the shared corner or edge (e.g. so that the M–X–M angle between corner‐shared polyhedra is less than 180°).
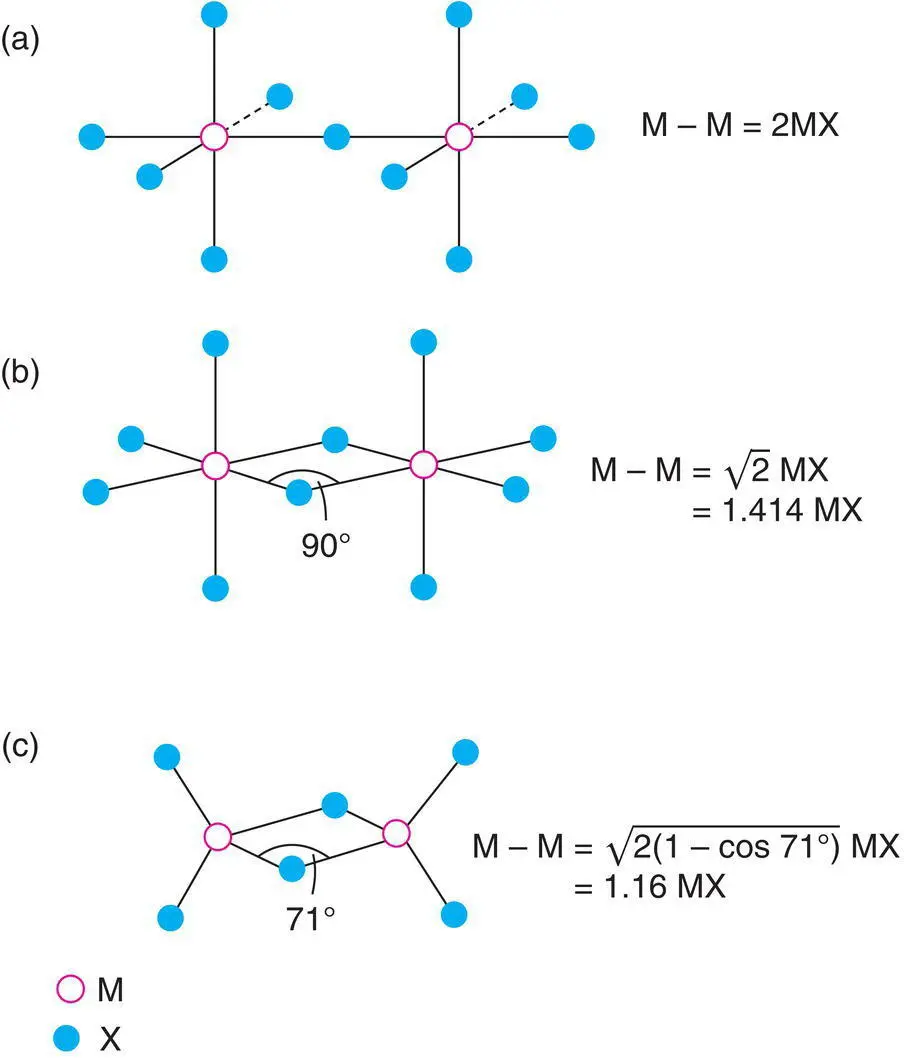
Figure 1.28 Cation–cation separation in octahedra which share (a) corners and (b) edges and in (c) tetrahedra which share edges.
From Table 1.6, the M–M distance in face‐shared tetrahedra is considerably less than the M–X bond distance. This therefore represents an unstable situation, because of the large cation–cation repulsions which would arise, and does not normally occur. A good example of the non‐occurrence of face‐sharing tetrahedra is provided by the absence of any crystal whose structure is the hcp equivalent of the fluorite (or antifluorite) structure. In Na 2O, which has the antifluorite structure, the oxide ions are in a ccp array and the NaO 4tetrahedra share edges. However, in a structure with an hcp anion array and all the tetrahedral cation sites occupied, the MX 4tetrahedra would share faces. Edge sharing of tetrahedra, in which the M–M separation is only 16% larger than the M–X bond distance (Table 1.6) is energetically acceptable, as shown by the common occurrence of compounds with a fluorite structure, but face sharing of tetrahedra is generally unacceptable.
Table 1.6 The M–M distance between MX4 or MX6 groups sharing X atom(s)
|
Corner sharing |
Edge sharing |
Face sharing |
| Two tetrahedra |
2.00 MX(tet.) a |
1.16 MX(tet.) a |
0.67 MX(tet.) |
| Two octahedra |
2.00 MX(oct.) a |
1.41 MX(oct.) a |
1.16 MX(oct.) |
a Maximum possible value.
For tetrahedra containing cations of high charge, edge sharing may be energetically unacceptable and only corner sharing occurs. For example, in silicate structures which are built of SiO 4tetrahedra, edge sharing of SiO 4tetrahedra never occurs. ( Note: In an ideally ionic structure, the charge on Si would be 4+, but the actual charges are considerably less due to partial covalency of the Si–O bonds.) In SiS 2, however, edge sharing of SiS 4tetrahedra does occur; the Si–S bond is longer than the Si–O bond and consequently the Si–Si distance in edge‐shared SiS 4tetrahedra is also greater and appears to be within the acceptable range for interatomic separations.
Читать дальше

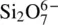
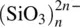 , chains, or rings
, chains, or rings











