1.17 Some Important Structure Types
1.17.1 Rock salt (NaCl), zinc blende or sphalerite (ZnS), fluorite (CaF 2), antifluorite (Na 2O)
These structures are considered together because they all have ccp/fcc anions and differ only in the positions of the cations. In Fig. 1.24 are shown the anions in a fcc unit cell with all possible O, T +and T –sites for the cations. There is no rule as to which sites should be labelled T +and T –; the choice is yours. The choice of origin is also arbitrary; for present purposes, it is more convenient to place the anions at the origin and also, therefore, at face centre positions. The different structures are generated as follows:
rock salt: O occupied; T+ and T– empty
zinc blende: T+ (or T–) occupied; O, T– (or T+) empty
antifluorite: T+, T– occupied; O empty.
Unit cells are shown in Fig. 1.29, in oblique projection (a–c) and as projections on the ab face of the unit cell in (d–f). Each is described in more detail later.
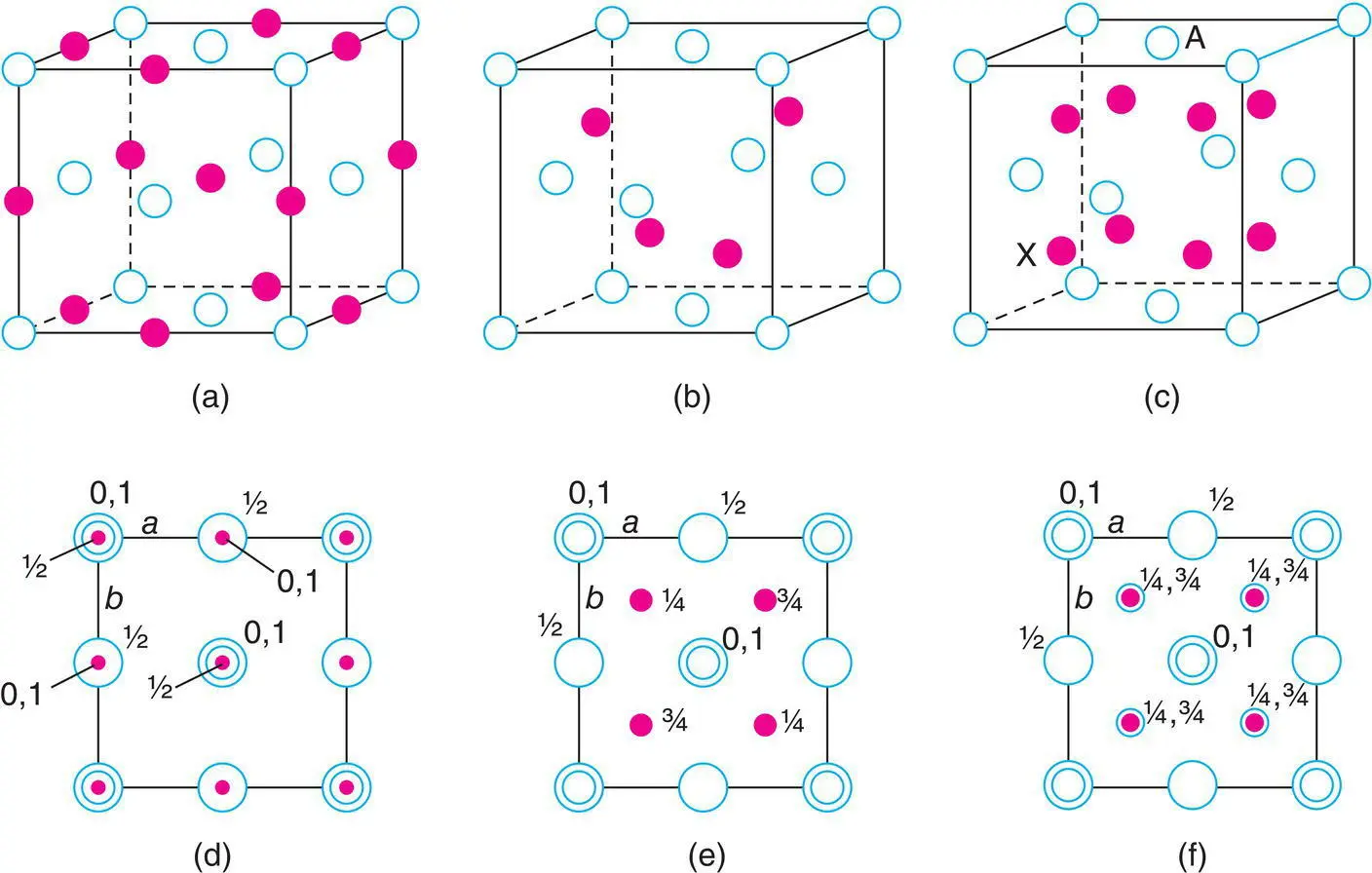
Figure 1.29 Unit cell of (a, d) NaCl, (b, e) ZnS, sphalerite, and (c, f) Na2O. Closed circles are cations; open circles are anions.
Table 1.7 Two ways to describe the antifluorite structure
|
Old cell |
New cell |
| Anions |
000, ½½0, ½0½, 0½½ |
¾¾¾, ¼¾¾, ¼¾¼, ¾¼¼ |
| Cations |
¼¼¼, ¼¼¾, ¼¾¼, ¾¼¼ |
000, 00½, 0½0, ½00 |
|
¼¾¾, ¾¼¾, ¾¾¼, ¾¾¾ |
0½½, ½0½, ½½0, ½½½ |
A general rule regarding coordination numbers is that in any structure of formula A xX y , the coordination numbers of A and X must be in the ratio of y:x . In both rock salt and zinc blende, x = y and therefore, in each, anions and cations have the same coordination number.
In antifluorite, of formula A 2X, the coordination numbers of cation and anion must be in the ratio of 1:2. Since the cations occupy tetrahedral sites, the anion coordination number must be eight. In order to see this, it is convenient to redefine the origin of the unit cell to coincide with a cation rather than an anion. This is done by displacing the unit cell along a body diagonal by one‐quarter of the length of the diagonal. The cation at X in Fig. 1.29(c), with coordinates ¼¼¼, may be chosen as the new origin of the unit cell. The coordinates of all atoms in the new cell are given by subtracting ¼¼¼ from their coordinates in the old cell, as in Table 1.7.
In cases where negative coordinates occur as a result of this subtraction, e.g. –¼–¼–¼, the position lies outside the new unit cell and it is necessary to find an equivalent position within the unit cell. In this particular case, 1 is added to each coordinate, giving ¾¾¾. Addition of 1 to, say, the x coordinate is equivalent to moving to a similar position in the next unit cell in the x direction. The new unit cell of antifluorite with its origin at cation X, is shown in Fig. 1.30(a). It contains cations at corners, edge centres, face centres and body centre.
In order to see the anion coordination more clearly, the unit cell may be imagined as divided into eight minicubes (as in Fig. 1.24). Each minicube in Fig. 1.30(a) has cations at all eight corners and at the centre of each is an eight‐coordinate site. Anions occupy four of these eight minicubes such that parallel to the cell axes the eight‐coordinate sites are alternately occupied and empty. The eightfold coordination for one anion, A, is shown in Fig. 1.30(b).
In the antifluorite structure, the effect of changing the origin from an anion to a cation is to show the structure in a completely different light. This does not happen with the rock salt and zinc blende structures. In these, the cation and anion positions are interchangeable and it is immaterial whether the origin coincides with an anion or a cation.
So far, the NaCl, ZnS and Na 2O structures have been described in two ways: (a) as cp structures and (b) in terms of their unit cells. A third way is to regard them as built of space‐filling polyhedra. Each ion and its nearest neighbours may be represented by the appropriate polyhedron, e.g. in zinc blende a tetrahedron represents one Zn with four S neighbours (or vice versa). It is then necessary to consider how neighbouring polyhedra are linked in 3D. Let us now consider each of these structures in more detail.

Figure 1.30 Alternative view of the antifluorite structure.
1.17.1.1 Rock salt structure
To summarise, the rock salt structure has ccp/fcc anions with octahedral sites fully occupied by cations and tetrahedral sites empty. Each cation is surrounded by six anions, as shown for cations 1, 2 and 3 in Fig. 1.31; similarly, each anion is octahedrally coordinated by cations (to see this, consider the anion at the top face centre, ½½1, which is coordinated to the four edge centre cations in the top face, to the cation at the body centre, shown, and to a sixth cation at the body centre of the unit cell above).
The (NaCl 6) or (ClNa 6) octahedra share common edges, Fig. 1.31. Since each has 12 edges and each edge is common to two octahedra, it is difficult to represent this satisfactorily in a drawing; Fig. 1.31 shows just two such linkages. A perspective which focuses on the 3D array of octahedra is shown in Fig. 1.32; the structure may be regarded as layers of octahedra, which also have an ABC stacking sequence similar to the anions. Each octahedron face is parallel to a cp layer of anions, as emphasised by the numbering or shading of coplanar faces. Parts of four different sets of faces are seen, corresponding to the four cp orientations in a ccp/fcc array. Also shown, arrowed, in Fig. 1.32 are the empty tetrahedral sites.
A large number of AB compounds possess the rock salt structure. A selection is given in Table 1.8 together with values of the a dimension of the cubic unit cell. Most halides and hydrides of the alkali metals and Ag +have this structure, as do a large number of chalcogenides (oxides, sulphides, etc.) of divalent metals. Many are ionic but others are either metallic, e.g. TiO, or covalent, e.g. TiC.
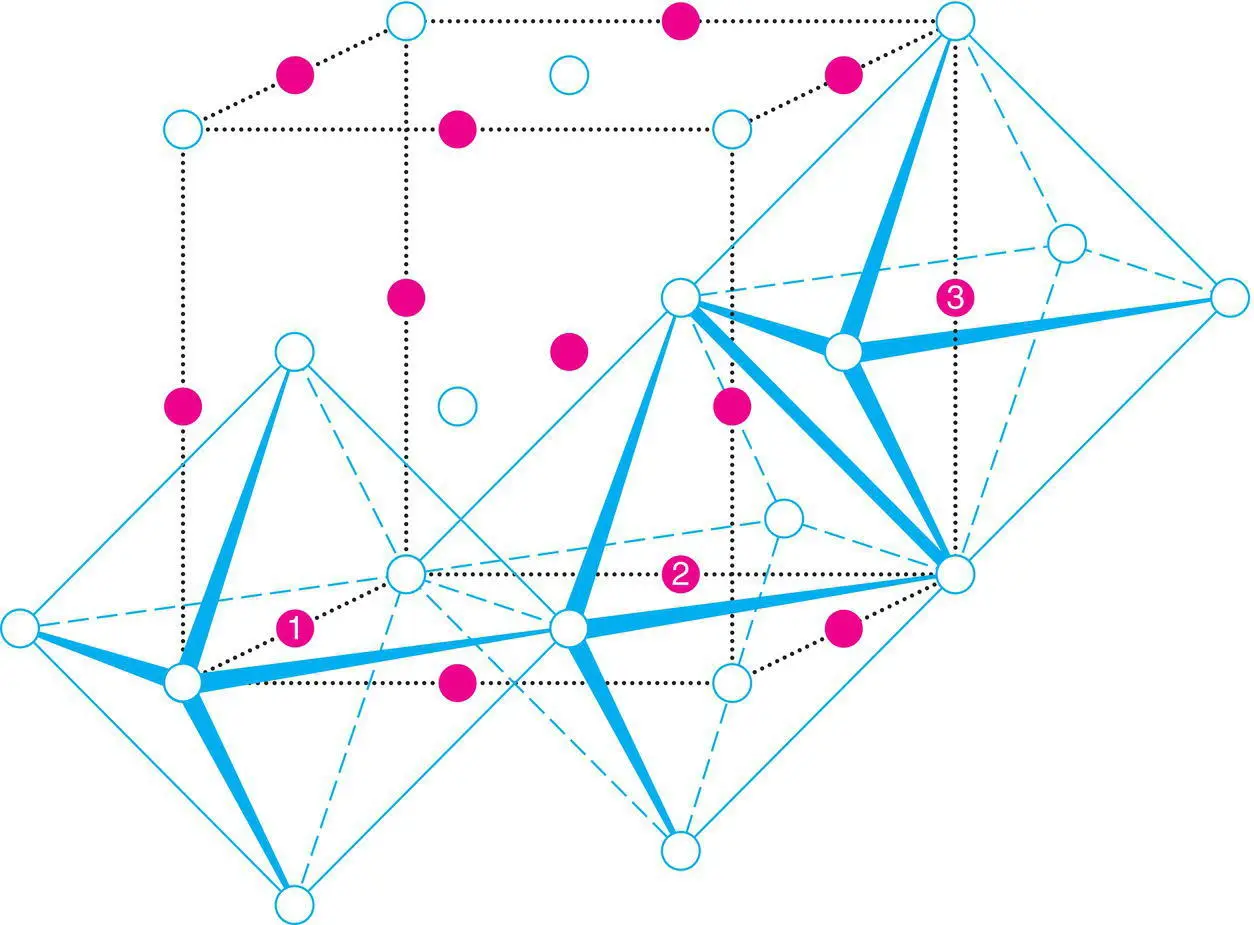
Figure 1.31 Unit cell of the rock salt structure showing edge‐sharing octahedra.
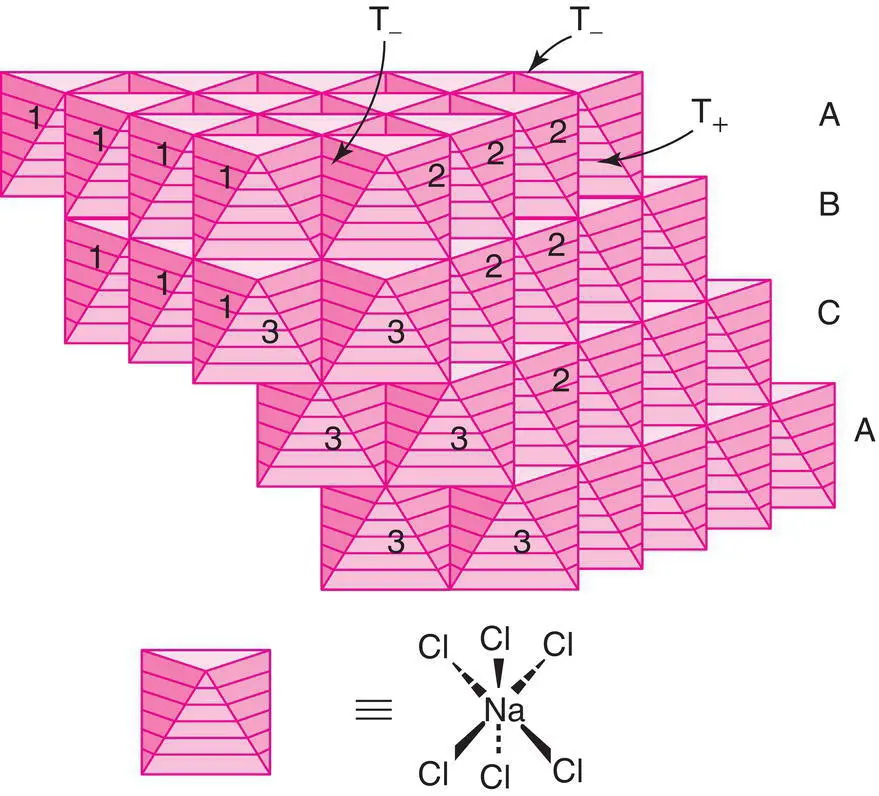
Figure 1.32 The rock salt structure as an array of edge‐sharing octahedra.
1.17.1.2 Zinc blende (sphalerite) structure
The zinc blende structure has ccp/fcc anions with cations in one set of tetrahedral sites, either T +or T –. The ZnS 4tetrahedra are linked at their corners and each corner is common to four such tetrahedra. The unit cell of zinc blende, Fig. 1.29(b), is shown again in Fig. 1.33(a), but in terms of corner‐sharing ZnS 4tetrahedra. The faces of the tetrahedra are parallel to the cp anion layers, i.e. the {111} planes; this is emphasised in a more extensive model in Fig. 1.33(b), which is oriented so that one set of tetrahedron faces is approximately horizontal. Conventionally, ZnS is regarded as built of cp layers of sulphides anions with the smaller zinc cations in tetrahedral sites. Since the same structure is generated by interchanging the Zn and S, the structure could also be described as a cp array of Zn with S occupying one set of tetrahedral sites. A third, equivalent description is as an array of ccp ZnS 4(or SZn 4) tetrahedra.
Читать дальше
















