1 ...7 8 9 11 12 13 ...44 Green AIEgens of donor– π –acceptor (D– π –A) systems, with intramolecular charge transfer (ICT) from donor end to acceptor, also attracted great attention in molecule design and found broad applications in construction of OLEDs [66]. Tang et al. took diphenylamino and dimesitylboryl groups as donor and acceptor, respectively, to combine with the TPE core, and a series of AIE‐based star‐shaped bipolar TPE derivatives TPE‐2PN2PB, TPE‐PN3PB, and TPE‐4PB( Figure 1.3) were prepared with PLQY of up to 95% in the solid state. Based on these emitters, nondoped OLEDs through solution process were successfully fabricated, with the structure of ITO (130 nm)/PEDOT : PSS (40 nm)/EML (70 nm)/TPBi (30 nm)/Ba (4 nm)/Al (120 nm). These OLEDs showed peak luminance and EQE of 11 665 cd/m 2and 2.6%, respectively [67]. In another research, they introduced carbazole moiety with hole‐transporting property and dimesityl boron groups with electron‐transporting property as peripheries, into TPE core, to obtain two green AIE emitters of PDPBCEand BDPBCE( Figure 1.3). Based on these two emitters, nondoped OLEDs were fabricated with the structure of ITO/HATCN (10 nm)/NPB (50 nm)/EML (30 nm)/TPBi (40 nm)/LiF (1 nm)/Al (150 nm). Both the devices showed green‐light emission, with a maximum brightness of 67 500 cd/m 2and a maximum CE of 11.2 cd/A [68]. Furthermore, they applied N ‐ethyl‐carbazole group, and dimesitylboron or (dimesitylboranyl)phenyl groups were attached TPE core and linked to the para‐ or meta‐position of TPE to obtain four AIE luminogens of p‐DPDECZ, p‐DBPDECZ, m‐DPDECZ, and m‐DBPDECZ( Figure 1.3). Compared to the meta‐linked, compound‐based blue OLEDs, the nondoped OLED devices with para‐linked compound exhibited better EL performance, with a maximum brightness of 30 210 cd/m 2and a maximum CE of 9.96 cd/A, due to the increased conjugated length [69].
1.2.3 Red Aggregation‐induced Emissive Emitters
Red emitters, as one indispensable part of the three‐primary‐color system, play an important role in the full‐color displaying and white‐light emitting systems. Therefore, various AIE emitters with different structures and high efficiency have been prepared and applied in the OLED area [70]. In addition, red AIEgens also found broad applications in biological areas, especially for in vitro and in vivo bioimaging, due to being less detrimental to tissues, deeper penetrating length into tissues, and lower overlapping with background fluorescence, compared with other chromatic emitters [71, 72]. However, compared to AIE luminogens with blue or green emission, the number of red and near infrared (NIR) emissive AIEgens are relatively smaller, and they also share the lower efficiency, due to two reasons: (i) low energy gap of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) in the red emitters resulting in high intersystem conversion (IC) and (ii) large dipole moments of donor and acceptor with a high tendency to self‐quench [70]. The red AIE emitters should have lower energy gap, and the main strategies to decrease the energy gap by expanding the π ‐conjugating length and/or strengthening the donor–acceptor interactions. Since most AIE emitters shared much more twisted molecular structure with shorter conjugating length, it could be observed from the red AIE emitters already published that most contained strong donor, such as triphenylamine, and acceptor, such as benzothiadiazole and cyano [73].
The moiety of heterocyclic benzo‐2,1,3‐thiadiazole ( TD) was a well‐known building block for red emitters due to its strong electron‐withdrawing properties. Zhao et al. took advantage of TPE, TD, and thiophene building blocks to construct two red emitters BTPETTDand BTPEBTTD( Figure 1.4), with strong built‐in push–pull interactions. OLED devices [ITO/NPB (60 nm)/EML (20 nm)/TPBi (10 nm)/Alq 3(30 nm)/LiF (1 nm)/Al (100 nm)] were fabricated based on these red emitters BTPETTDand BTPEBTTDthat exhibited emission at 592 and 668 nm, respectively, with maximum CE, PE, and EQE of 8330 cd/m 2, 6.4 cd/A, and 3.1% [74]. Furthermore, they modified BTPEBTTDby attaching an additional TPE unit to the thiophene moiety to obtain the AIE emitter of TTPEBTTD( Figure 1.4), finding a dramatic red‐shift of the emission wavelength. Based on this emitter, the nondoped OLED devices exhibited red emission peaking at 650 nm (CIE = 0.67, 0.32), with CE and EQE of 3750 cd/m 2and 3.7%, which is higher than the BTPEBTTD‐based OLED device, as a result of weakened intermolecular interaction [75]. Xu et al. prepared two new AIE luminogens V2BV2and T2BT2( Figure 1.4), composed of TD cores, TPA bridges, and AIE‐active end‐capper. With multiple TPE units, the luminogens exhibited AEE (aggregation‐enhanced emission) properties. The nondoped OLED devices ITO/NPB (60 nm)/sample (20 nm)/TPBi (40 nm)/LiF (1.0 nm)/Al (100 nm) were fabricated with emission of 590 nm for T2BT2and 616 nm for V2BV2, and the maximum PE was found at 6.81 cd/A and 4.96 lm/W for the T2BT2device [76]. Qin et al. utilized the moieties of TPE, TD and arylamines triphenyal amine, or N , N ′‐di(1‐naphthyl)‐ N , N ′‐diphenyl‐(1,1′‐biphenyl)‐4,4′‐diamine ( NPB) to two novel red AIE‐active emitters TPE‐TPA‐BTD( TTB) and TPE‐NPA‐BTD( TNB) ( Figure 1.4), which can be used as EMLs or both HTLs and EMLs for OLEDs with good EL performance. The nondoped OLED based on these emitters as EML can obtain EQE of up to 3.9%, while the bilayer OLED using TTB as HTLs and EMLs can reach EQ of up to 2.5%, with much simpler OLED structure [77]. Wang and coworkers prepared two new red AIE emitters composed by the stronger electron‐deficient core of [1,2,5]thiadiazolo[3,4‐ g ]quinoxaline or benzo[1,2‐c; 4,5‐c′] bis [1,2,5]thiadiazole and TPE end‐cappers. The resulting luminogens TDQand BBT( Figure 1.4) show the AEE effect and their films emit NIR PL and EL emissions peaked in the range of 704–883 nm. Efficient nondoped NIR OLEDs were achieved using these materials as emitting layers, with a maximum EQE of 0.89% [78].
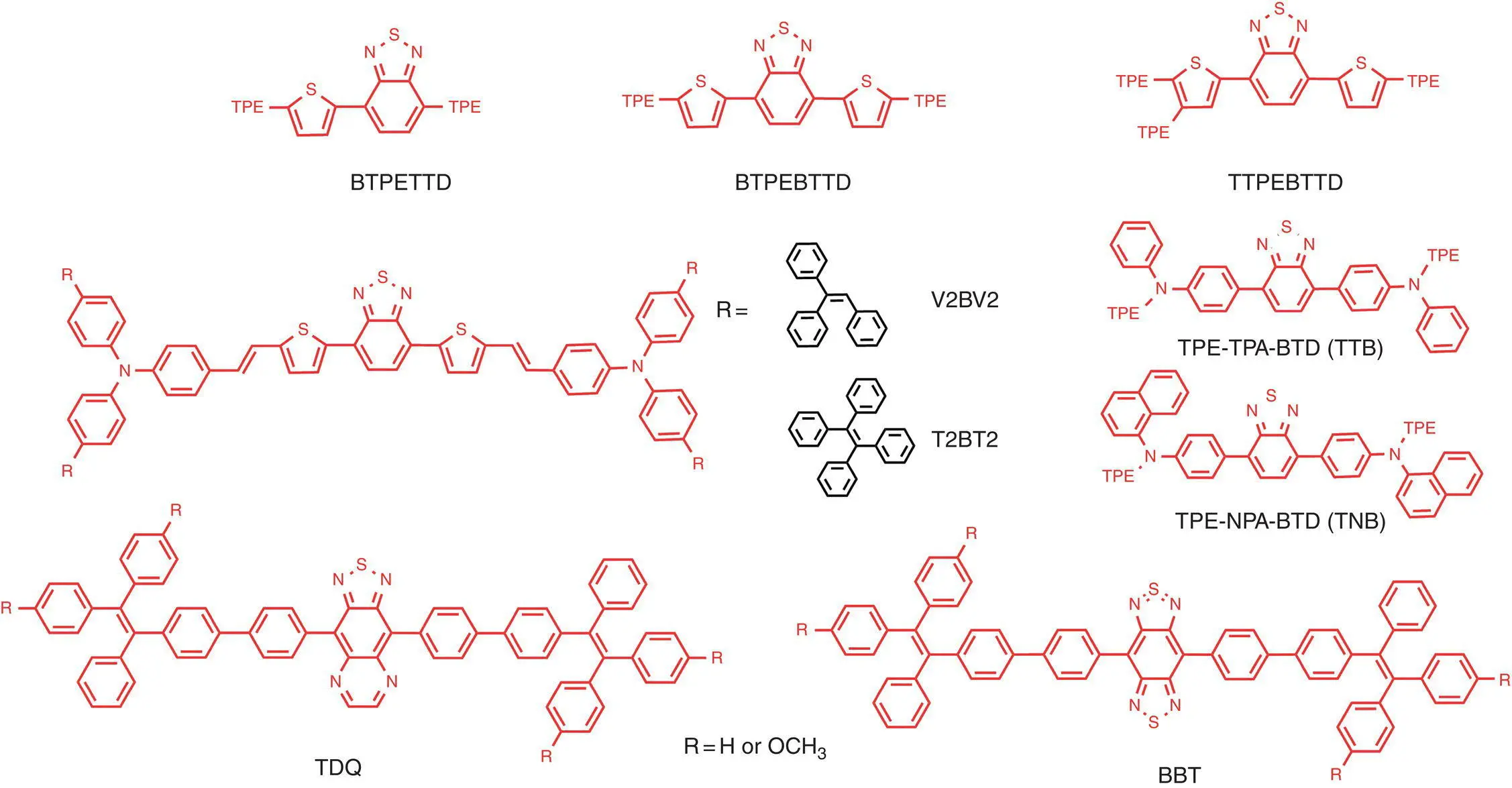
Figure 1.4 Molecular structures of red conventional AIE‐active emitters.
1.2.4 Aggregation‐induced Emission‐active Emitters‐Based White OLED
White organic light‐emitting devices (WOLEDs) have attracted significant research interest both in academia and industry due to their potential application in solid‐state lighting and full‐color displays. Till now WOLEDs were usually obtained by blending the emission from two‐colored (blue and yellow) or three‐colored (red, green, and blue) emitters. One possible solution to preparing WOLEDs is to dope the emitters into a wide band‐gap host to overcome the ACQ effect. Tang et al. reported a novel blue‐emissive AIE material BTPE( Figure 1.5) with wide band gap of 3.1 eV. Based on this material as the host and the commercially available emitters DCJTBdoped as the EML, the related WOLEDs could reach color rendering index, maximum luminance, and CE of 84, 10 319 cd/m 2, and 7 cd/A, respectively, with CIE coordinate of (0.36, 0.38) [79].
Читать дальше













