1 ...6 7 8 10 11 12 ...44 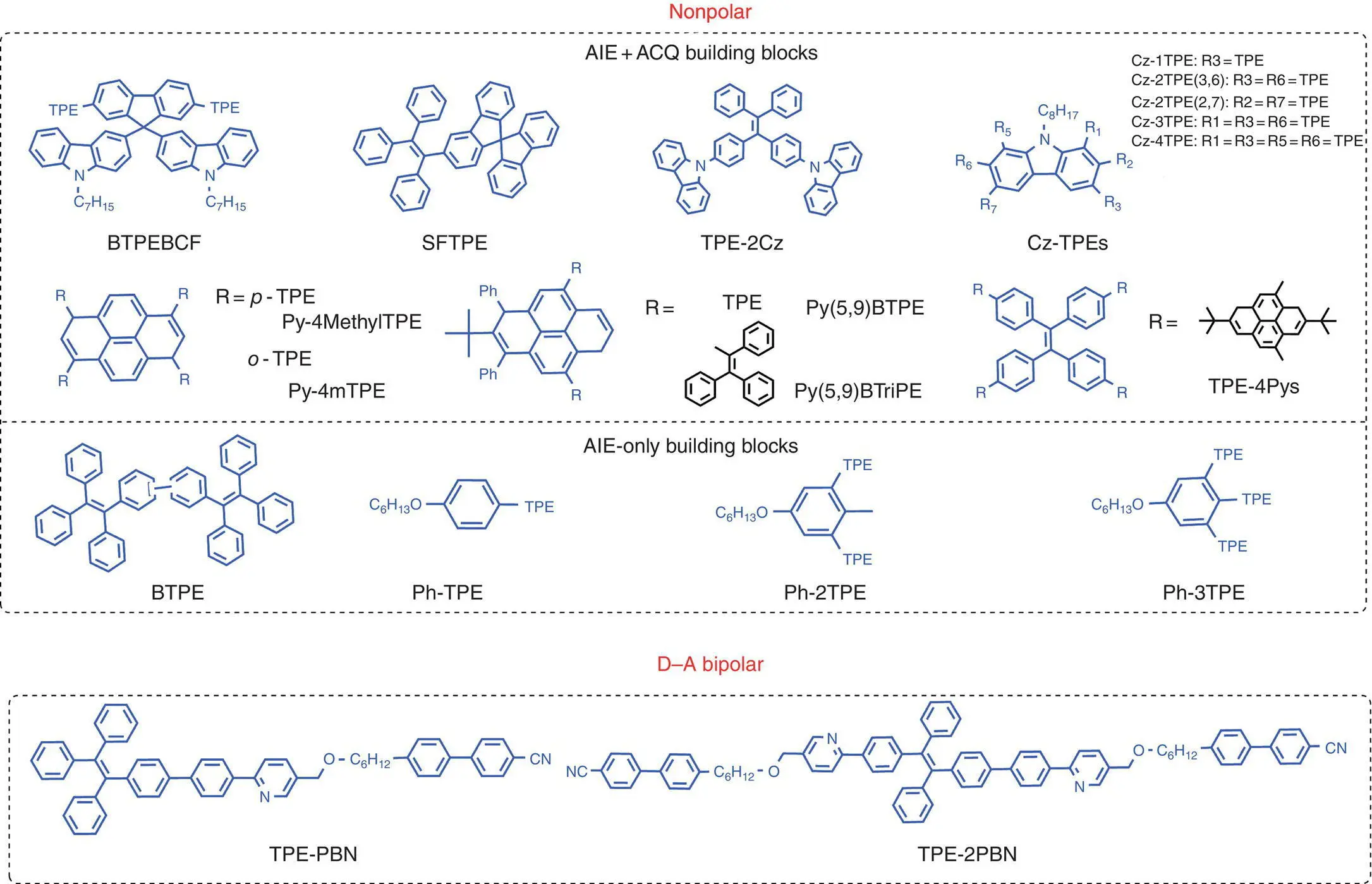
Figure 1.2 Molecular structures of TPE‐based conventional blue AIE‐active emitters.
In some other works, TPE served as single fluorescent moieties to construct more complicated AIE emitters. Li et al. merged two TPE units together through linking at different positions to obtain four BTPE( Figure 1.2) derivatives with deep‐blue emission. Nondoped sky‐blue OLED devices with a configuration of ITO/MoO 3(10 nm)/NPB (60 nm)/EML (15 nm)/TPBi (35 nm)/LiF (1 nm)/Al (100 nm) were fabricated, with maximum luminance, CE, and PE of 3266 cd/m 2, 2.8 cd/A, and 2.0 lm/W, respectively [50]. They further decorated the benzene core with different number of TPE moiety peripheries to obtain three AIE emitters PhTPE, Ph2TPE, and Ph3TP( Figure 1.2), based on which nondoped blue OLEDs were fabricated with the structure of ITO/MoO 3(10 nm)/NPB (80 nm)/EML (20 or 30 nm)/TPBi (30 nm)/LiF (1 nm)/Al, with emission ranging from 457 to 488 nm, and maximum CE, luminance, and PE of 5.0 cd/A, 3966 cd/m 2, and 3.87 lm/W, respectively [51].
On the other hand, as an important factor, balanced factor of carriers’ transport (electrons and holes) can also decide the overall electroluminescent performance. However, owing to the electron‐rich nature of most organic materials, they exhibit high hole transport but relatively low electron mobility, and finally lead to unbalanced electron‐hole recombination. In this regard, bipolar materials with donor−acceptor (D−A) structures can favor both holes and electrons injection and transport to result in balanced factor of carriers’ transport (electrons and holes), which finally enhance the efficiency of the OLEDs. Additionally, the dipolar luminogens tend to align horizontally in solid state, which can also result in high out‐coupling efficiency over 20%. In previous research, most dipolar emitters were served as host or dopant, because the strong D−A interaction usually showed lower PL efficiency at solid state [52, 53]. For example, Xie et al. designed and prepared two novel AIE materials of TPEPBNand TPE‐2PBN( Figure 1.2) with the property of liquid crystal due to 4‐cynobiphenyl moiety serving as the mesogenic unit, and the PLQYs can be obtained 71 and 83% in solid state, respectively. Due to liquid crystals of electron‐accepting moiety of CN, these both compounds showed high hole mobility. Based on these two AIE emitters, both nondoped and doped OLED devices were fabricated and the better OLED device were obtained for nondoped ones based on the emitter of TPE‐PBN( Figure 1.2), with maximum EQE of 4.1%, which is one of the highest values reported for blue fluorescent OLEDs base on AIE emitters [54].
Silole derivatives, with both good electron affinity and electron mobility, due to its σ *– π * conjugated electronic structures, also exhibit the propeller‐like molecular structures and therefore the property of AIE, which also attracted the research interest in taking this moiety into the OLED emitters’ design. Li et al. covalently incorporated TPE moiety to a dibenzosilole core through three different link modes to obtain compounds Si‐pTPE, Si‐tPE, and Si‐mTPEwith light emitting from green to deep blue. The nondoped OLED based on Si‐tPEcan reach the maximum CE and EQE of 8.04 cd/A and 3.38%, respectively [55]. They also linked the TPE moiety to tetraphenylcyclopentadiene ( TPCP) with substitution of Si of silole by C to prepare six novel AIEgens with blue emission, and the nondoped OLED device can reach the maximum luminance and CE of 8721 cd/m 2and 3.40 cd/A, respectively. Recently, Tang et al. reported a new deep‐blue AIE emitter of tetraphenylbenzosilole ( TPBS), with similar structure to 1,1,2,3,4,5‐hexaphenylsilole ( HPS), based on which solution‐processed nondoped OLEDs were prepared, with EQEs of 3.6% and CIE coordinates of (0.15, 0.10) as the best spin‐coating type of AIEgens of nondoped OLEDs reported so far [56].
Tetraphenylpyrazinyl ( TPP) was a novel blue‐emissive AIE emitter first found in 2015 [57, 58]. Based on this moiety as electron donor and imidazole as electron acceptor, two deep‐blue AIE emitters of TPP‐PPI and TPP‐PI were prepared, with the property of planarized intramolecular charge transfer (PLICT). The nondoped blue OLEDs based on these emitters ( TPP‐PPIand TPP‐PI) were prepared showing good performance, with maximum EQE of 4.85 and 4.36% [59]. Also nonpolar blue emitter of TPP was further modified with carbazole, and its nondoped OLEDs exhibited optimal performance, with CIE coordinates of (0.16, 0.11) and maximum EQE of 1.49% [60].
1.2.2 Green Aggregation‐induced Emissive Emitters
In comparison to their blue and red counterparts, green AIEgens are quite facile to design, with suitable conjugated length and polarity. In this section, we exemplify the green AIE emitters sorted by nonpolar and polar emitter. Tanyeli et al. attached three peripheral moieties of indole, tert‐butylcarbazole, and tetrahydrocarbazole to the TPE core, to obtain TIPE, TTBCPE, and TDCPE( Figure 1.3), respectively, through two facile steps. Based on these emitters, nondoped green OLED devices with different constructions could be prepared with a maximum brightness, CE, and EQE of 18 000 cd/m 2, 7.7 cd/A, and 3.2%, respectively [61]. By the same method, they further took advantage of another four N ‐heteroaromatic rings to attach the TPE cores, and the AIE emitters were therefore prepared, based on which green OLED devices could be prepared and exhibited relatively lower EL performance, with brightness, CE, and EQE of 2600 cd/m 2, 3.6 cd/A, and 1.5%, respectively [62]. Similarly, Tang et al. used the well‐known and hole‐transporting materials of NPB to covalently integrate to the TPE core, with the resulted TPE‐NPBas a green AIEgen ( Figure 1.3). Using TPE‐NPBas emitters, nondoped green OLED devices were fabricated, with maximum luminance and CE of 11 981 cd/m 2and 11.9 cd/A, respectively [63]. Still some other nonpolar green emitters were reported. Lu and Wang et al. tethered 2‐aryl‐3‐cyanobenzofuran fluorophore with TPE core via 5‐, 6‐, and 7‐position of 2‐aryl‐3‐cyanobenzofuran, and its nondoped OLED devices were prepared with the structure of ITO/PEDOT : PSS (40 nm)/EML (60 nm)/TBPI (25 nm)/Ca (10 nm)/Ag (80 nm), where 6‐position substituted emitter‐based OLEDs exhibited the best performance with brightness, CE, PE, and EQE of 620 cd/m 2, 1.62 cd/A, 0.73 lm/W, and 0.63%, respectively [64]. Silole moiety was also adopted as effective building units to construct the green AIE‐active emitters, simply by increasing the conjugating length. For example, Zhao et al. designed and prepared three novel green‐emissive AIE‐active silole derivatives 2,2′‐MTPS‐CaP, 3,3′‐MTPS‐CaP, and 9,9′‐MTPS‐CaP( Figure 1.3) by connecting silole and electron‐donating groups of 9‐phenyl‐9 H ‐carbazole through different linkage. And the OLEDs based on the emitter 9,9′‐MTPS‐CaPhad the best EL performance, with the EQE of 5.63%, above the limit of 5% in traditional fluorescent OLEDs [65].
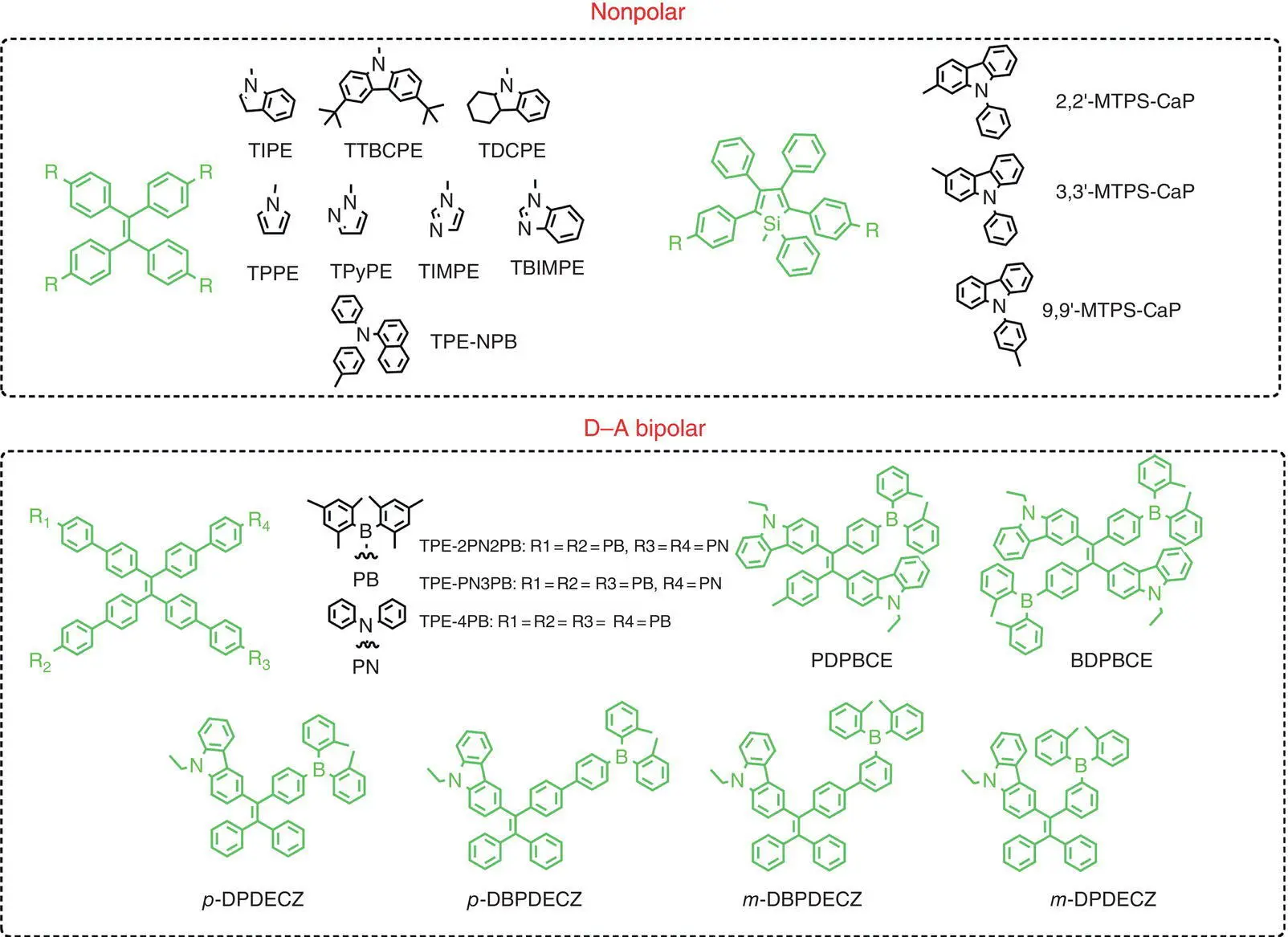
Figure 1.3 Molecular structures of green conventional AIE‐active emitters.
Читать дальше














