3.3.2 Brain MRI with a Portable Device
A portable MRI can be brought to the patient rather than bringing the patient to the MRI. Thus we define “portable” as a device that can be moved room-to-room by a single healthcare worker for use on a patient within minutes. The power and cooling infrastructure requirements must be met by a typical office or exam room infrastructure. Such portable MRI scanners equivalent to their counterparts in US, X-ray, and CT have traditionally not been available, although a commercial clinical portable MRI product has recently been introduced and tested in a clinical setting [36].
True portability seems to require both restriction to a limited body region (e.g. the head) and operation at low field (likely below 200 mT) and potentially employing new types of magnet architectures and encoding strategies. Here attention has turned to permanent magnets, which offer the ability to operate without external power or a cryogenic system. Modern rare-earth permanent magnets can produce head-sized B 0field regions up to about 0.5 T with surprisingly small external field footprints. Many magnet and gradient configurations have emerged, with several prototypes being demonstrated. Notably the first fully portable clinical MRI product scanner is now Food and Drug Administration (FDA) approved for clinical care – the 64-mT Hyperfine scanner [36,37]. Figure 3.2 shows another such system under development, based on an 80-mT “Halbach bulb” rare-earth magnet configuration with a magnet weight of 24 kg [38].
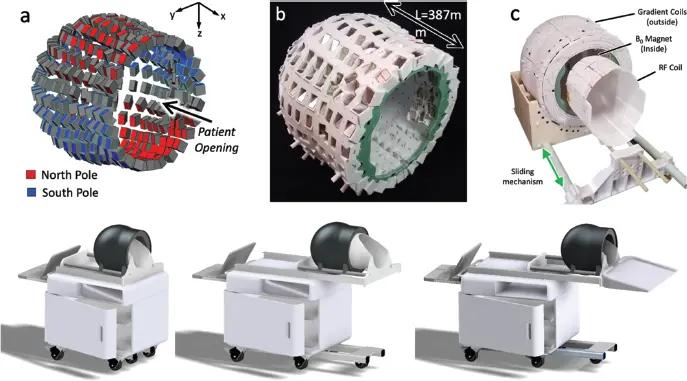
Figure 3.2 A potential portable brain magnetic resonance imaging cart based on a “Halbach bulb” permanent magnet array for bedside use in the emergency department or intensive care unit. Prototype magnet, gradient, and radiofrequency (top) and envisioned mobile cart (bottom); system approaches bed from head end and utilizes a double sliding mechanism (radiofrequency coil slides followed by magnet sliding down).
3.3.3 Brain MRI as a Monitoring Device
In addition to employing permanent magnets and reducing the magnet field strength and size, we also consider more extreme changes to the scanner architecture to further reduce the scanner size toward a handheld device. This includes dropping the goal of whole-organ imaging, for example probing only a section of the body with limited image encoding (2D, 1D, or even no image encoding). But, even if detailed anatomy is not visualized, the MR data can be collected and monitored for changes that might accompany, for example, important intracranial pathology. This type of MRI is thus a patient-monitoring device in the way a pulse-oximeter or electrocardiogram (ECG) device is used at the bedside in the ED or ICU. It is new territory for MRI and pushes the technology into a more radical configuration. This extreme approach does not attempt to have the system fully encircle a body part, but is limited to a “single-sided” approach following that originally introduced for materials characterization with MR [39,40] but also considered for biological applications [41–43] and using remarkably inexpensive components [44]. Single-sided spectrometers have also been introduced for assessing breast tissue [45,46] and muscle hydration [47,48]. Single-sided full-imaging systems are less common, but have been demonstrated [49] and applied to burn depth [50].
Figure 3.3 shows a single-sided scanner designed for brain imaging [51]. An ultra-lightweight (<10 kg) single-sided brain scanner will have extreme inhomogeneity by medical MRI standards and require unconventional encoding strategies. But these changes are likely necessary to make the leap from a scanner where the patient’s head still goes inside the device, to a more handheld scanner simply placed adjacent to the head. This step will not be without image quality reductions. For monitoring, the device must “reach” into the bed, operate adjacent to the patient (versus placing the anatomy inside the magnet), and be light and cheap enough for sustained operation as a monitoring device. An ED or ICU might benefit from such an MRI device to continuously image the brain watching for intracranial hemorrhage or changes in cerebral mass effect though monitoring a ventricular/cerebrospinal fluid left–right hemisphere asymmetry. An intracranial MR monitor could provide an early warning sign of impending herniation, particularly in patients where clinical examination is difficult (e.g. sedated patients).
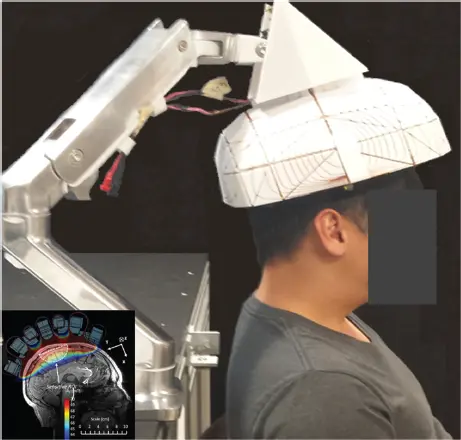
Figure 3.3 Single-sided brain magnetic resonance imaging under development for monitoring applications in the emergency department or intensive care unit. The <7 kg Halbach sphere-section magnet generates ~80 mT in a subregion of the brain.
3.4 Clinical Use Scenarios of “Easy-to-Site” POC, and Monitoring MR Devices
Although engineers and researchers are often content with the “build it and they will come” philosophy, clinicians, funding agencies, and hospital administrators prefer a need-driven approach. Furthermore, knowledge of the clinical use is critical to many design decisions. Here we frame the technical needs by outlining some of the clinical use scenarios envisioned for POC neurological MRI.
Neuroimaging in emergency medicine often focuses on immediately ruling out or identifying a source of increased ICP from hemorrhage, stroke, hydrocephalus (enlargement of the ventricles), or cerebral mass effect (displacement of brain tissue by blood or tumor or following trauma). This is currently done in the ED using CT scanners, which are fast, relatively cheap, and easy to site compared with MRI. The addition of POC MRI would be helpful for assessment of neurological emergencies for which CT provides a poor depiction or for patient populations sensitive to the ionizing radiation used in CT.
The use of low-field MRI in the stroke setting has recently been reviewed [52] including for resource-limited settings [53]. Portable low-field MRI has been contemplated for ruling out a hemorrhagic stroke to allow immediate reperfusion with the injection of recombinant tissue plasminogen activator (rtPA), which would exacerbate any existing cerebral hemorrhage. However, CT currently performs this job well, including portable units in EDs and even specialized CT-equipped ambulances [54–58] dispatched when stroke is suspected. Although a portable MRI would also be capable of this “rule out a bleed” role, it is worth looking to the next step of stroke care to avoid simply replacing one capable mobile imaging modality for another. Successful new intravenous thrombolytic therapies as well as widening windows for treatment have led to catheter-based thrombectomy treatments following the initial rtPA treatment [59,60]. A “hub-and-spokes” model for treatment sites has emerged for this treatment. First, patients with suspected stroke are rushed to the nearest “spoke hospital” where they receive a CT to verify stroke and rule out hemorrhage for immediate administration of rtPA. In the few cities with CT-equipped ambulances (mobile stroke units), this treatment is received in the field. Following rtPA administration, the patient is then evaluated for transfer to a “hub” hospital for catheter-based thrombectomy [59,60]. This assessment uses CT angiography (CTA) to identify a large vessel occlusion (LVO) target in the anterior circulation and perfusion (CTP) to estimate infarct size. An infarct core size >70 cc both reduces the likely benefit of revascularization and establishes a risk for hemorrhage during the procedure. Unfortunately, the infarct size determination is difficult and imprecisely measured on CTP [61]. In contrast, diffusion MRI is the established gold standard for early infarct core volume assessment [61] but is not commonly available in EDs [62,63]. The time-critical nature of acute stroke care and the cost of transport underscore the need for improved POC MR imaging in the spoke hospital ED to determine the eligibility of the patient for transport to receive catheter-based thrombectomy.
Читать дальше








