Abbreviations for ribonucleosides and 2-deoxyribonucleosides are derived from those used for the bases plus those for the ribosyl or 2′-deoxyribosyl groups. Thus AR stands for adenosine and AdR is the abbreviation for deoxyadenosine. In the case of inosine, (hypoxanthine + ribose) HR may be possible, but IR is often used. The latter is used in this text.
For nucleotides, the traditional abbreviations based on the term ‘nucleoside monophosphate’ are used. Thus AMP stands for adenosine monophosphate (adenylate), UMP for uridine monophosphate (uridylate), and NMP for any ribonucleoside monophosphate. Similarly, dAMP stands for deoxyadenosine monophosphate (deoxyadenylate), dUMP for deoxyuridine monophosphate (deoxyuridylate), and dNMP for any deoxyribonucleoside monophosphate. Thymidine monophosphate (thymidylate) often does not have the ‘deoxy’ prefix in its name, because thymidine is thymine deoxyriboside. However, the symbol including a ‘d’ is commonly used in biochemistry textbooks, so dTMP is adopted in this article.
1.3 Chemical Structures of Nucleotide-Related Compounds
Studies on purines and pyrimidines began in 1776 when the Swedish pharmacist Carl Wilhelm Scheele isolated uric acid from bladder stones. In 1846, Unger isolated guanine from the guano of Peruvian sea birds. At the end of the nineteenth century, several purines (adenine, xanthine, and hypoxanthine) and pyrimidines (thymine, cytosine, and uracil) were discovered by the German biochemist, Albrecht Kossel who believed they constituted the main part of cell nuclei. In 1874 Friedrich Miescher isolated nuclear material rich in phosphorus which he called ‘nuclein’. In the same period, Emil Fischer (1884) elucidated the structures of caffeine and related compounds which he confirmed by chemical synthesis. Further information can be found in a historical survey by Burnstock and Verkhratsky (2012). The pyridine nucleotide, NAD was discovered by the British biochemists Arthur Harden and William John Young in the early twentieth century (Harden and Young 1906).
A purine is a heterocyclic compound that consists of a pyrimidine ring fused to an imidazole ring. The word, ‘purine’ (‘Purum’ + ‘Uricum’) was coined by Emil Fischer (1884).
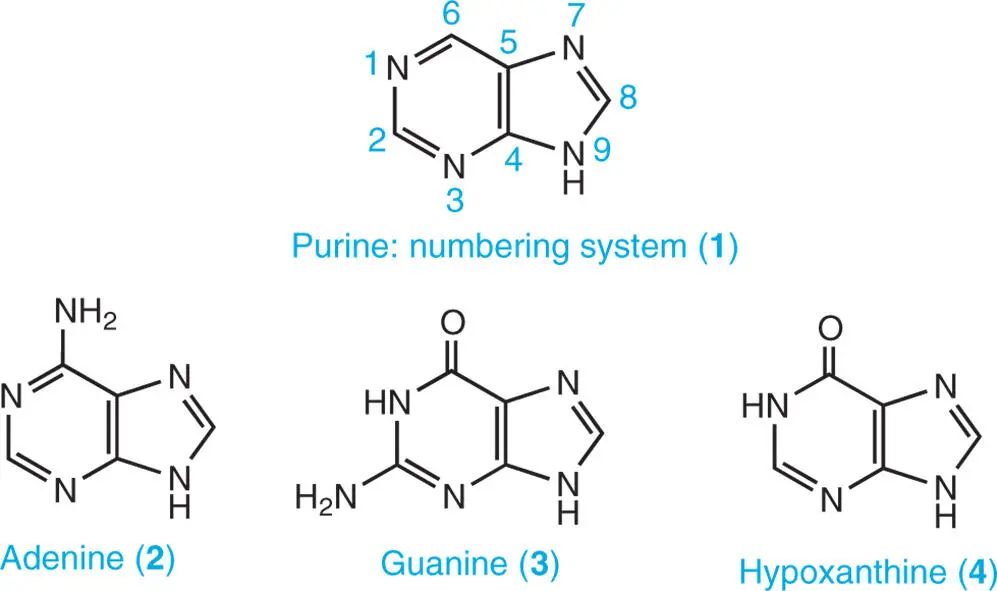
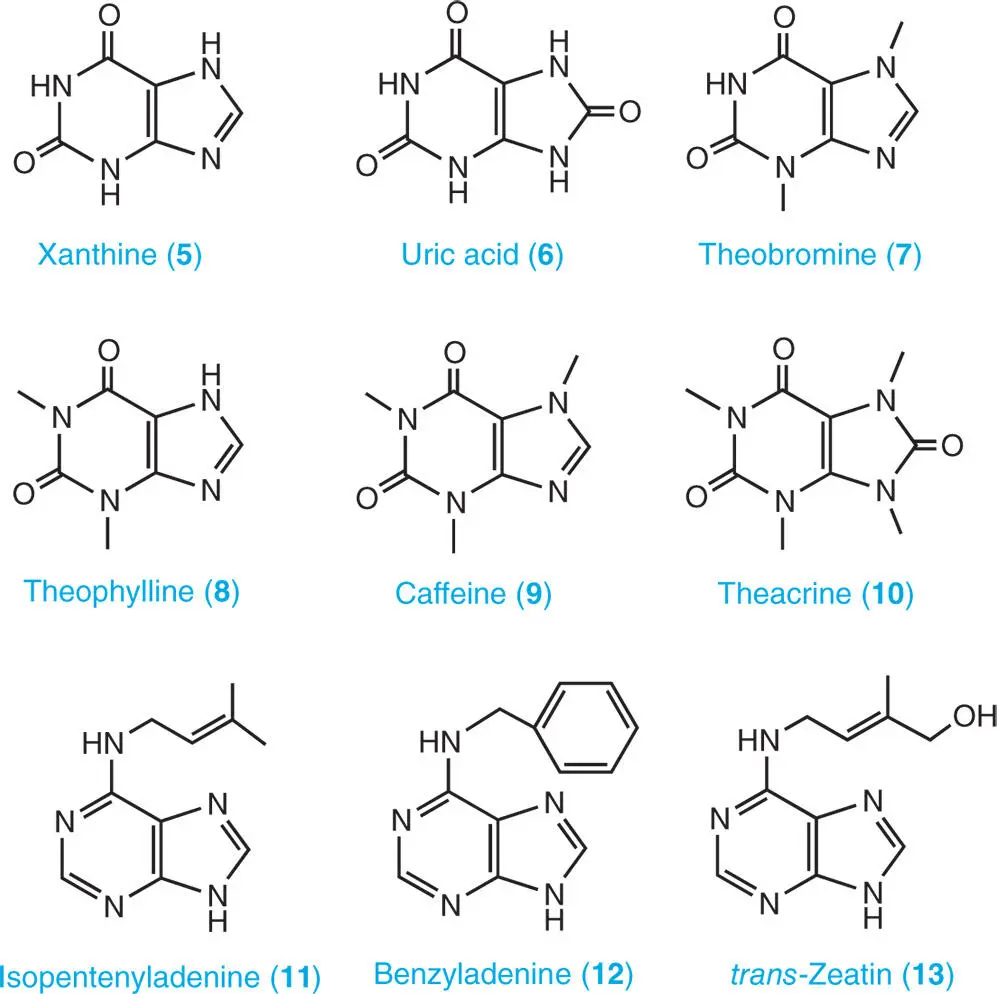
As shown in structure 1the atoms of the purine ring are numbered in an anticlockwise manner. In plants, there are several naturally occurring purine bases. They include adenine ( 2) and guanine ( 3), which are constituents of nucleic acids, and hypoxanthine ( 4), xanthine ( 5), and uric acid ( 6), which are produced as catabolites of adenine and guanine. Purine alkaloids, such as theobromine (3,7-dimethylxanthine) ( 7), theophylline (1,3-dimethylxanthine) ( 8), caffeine (1,3,7-trimethylxanthine) ( 9), and theacrine (1,3,7,9-tetramethyluric acid) ( 10) are derived from purine nucleotides, as are the major cytokinin plant hormones isopentenyladenine ( 11), benzyladenine ( 12), and trans -zeatin ( 13) (Ashihara et al. 2013).
1.3.1.2 Purine Nucleosides
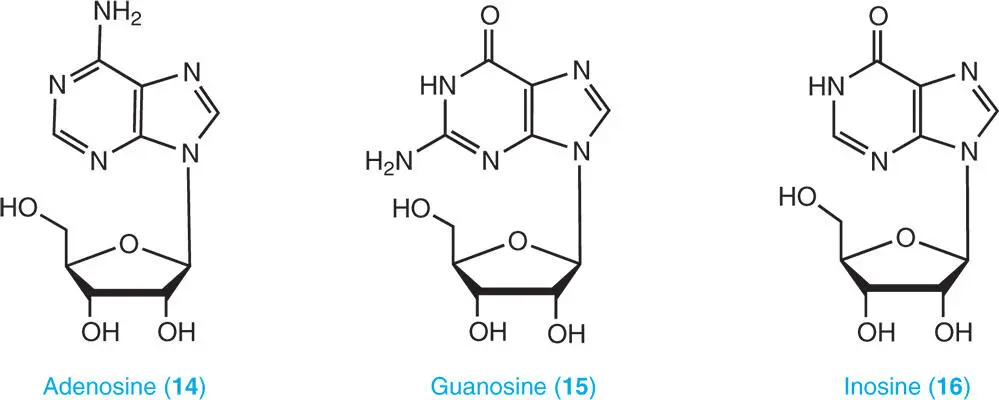
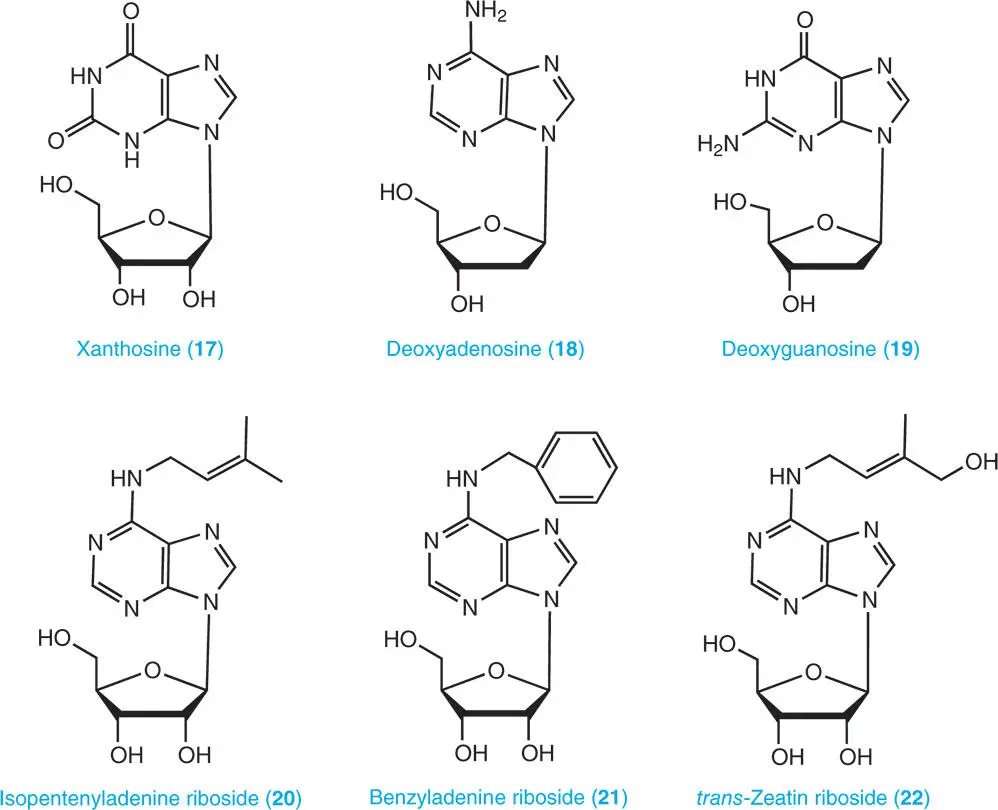
A nucleoside consists of a nucleobase and a five-carbon sugar (ribose or deoxyribose). Adenosine ( 14), guanosine ( 15), inosine ( 16), and xanthosine ( 17) are catabolites of purine ribonucleotides and RNA while deoxyadenosine ( 18) and deoxyguanosine ( 19) are catabolites of DNA. Cytokinins also occur as ribosides, namely, isopentenyladenine riboside ( 20), benzyladenine riboside ( 21), and trans -zeatin riboside ( 22) (Ashihara et al. 2013).
1.3.1.3 Purine Nucleotides
A nucleotide is composed of a purine base, a sugar moiety (ribose or deoxyribose) and at least one phosphate group. The phosphate group is attached to either the C3′or C5′ position of the sugar. Nucleoside-5′-phosphates (5′-nucleotides) are the main purine nucleotides in plants as well as other organisms. Small nucleoside-3′-monophosphate (3′-nucleotides) pools are mainly produced as catabolites of nucleic acids. Examples of different forms of nucleotides are adenosine-5′-monophosphate (5′-AMP, usually abbreviated as AMP) ( 23), adenosine-3′-monophosphate (3′-AMP) ( 24), deoxyadenosine-5′-monophosphate (dAMP) ( 25), and isopentenyladenine ribotide ( 26).
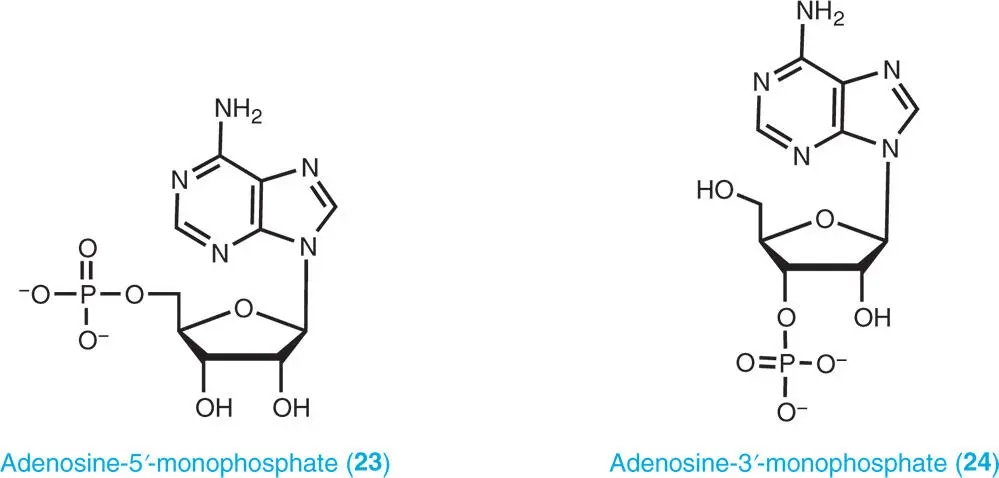
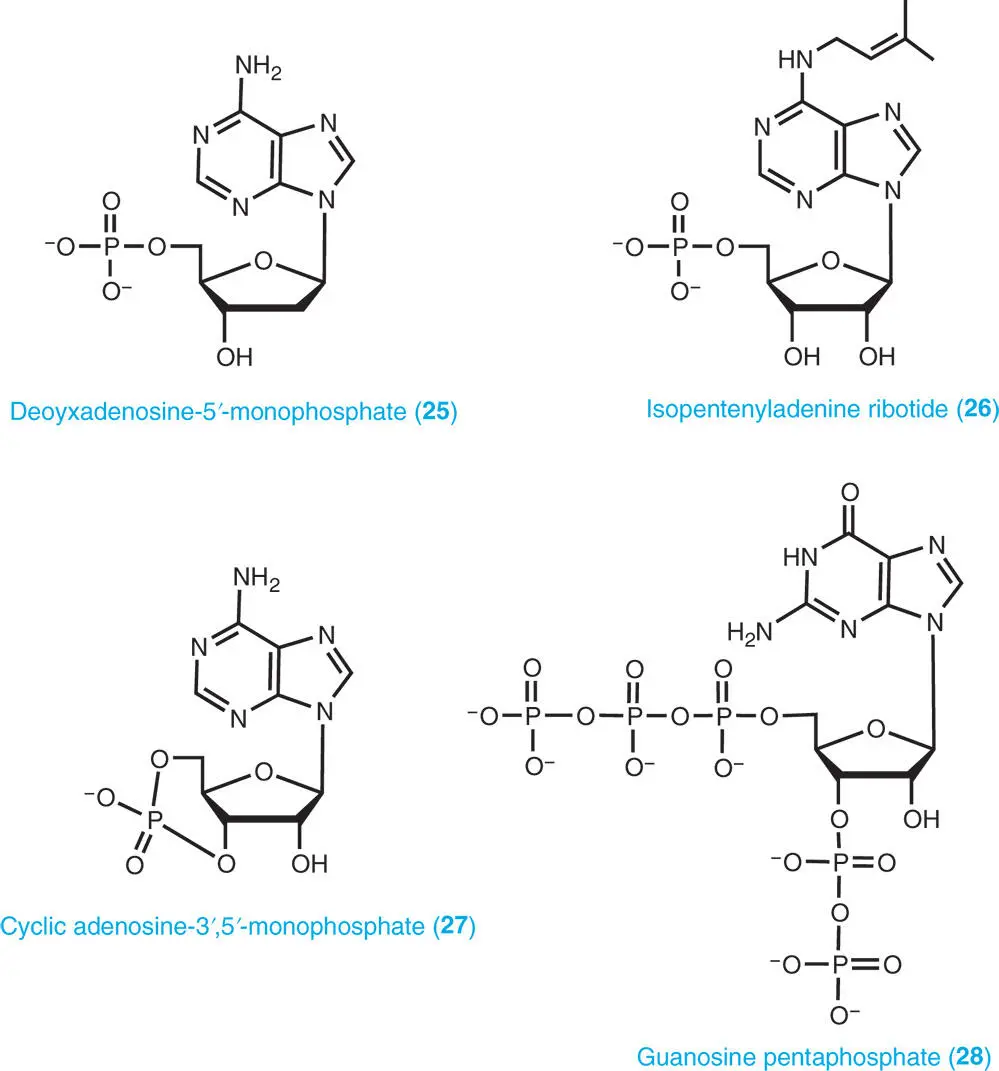
The cyclic monophosphates, cyclic adenosine-3′,5′-monophosphate (cAMP) ( 27) and cyclic guanosine-3′,5′-monophosphate (cGMP) occur in plants. These cyclic nucleotides are derived from ATP and GTP and act as second messengers. In addition, some unusual nucleotides, such as guanosine tetraphosphate (ppGpp), guanosine pentaphosphate (pppGpp) ( 28), diadenosine triphosphate (Ap 3A), and diadenosine tetraphosphate (Ap 4A), known as alarmones, which act as intracellular signal molecules, are produced in response to harsh environmental conditions (Boniecka et al. 2017; Pietrowska-Borek et al. 2011). Possible roles of these unusual nucleotides in plants are outlined in Part VIII.
Pyrimidine ( 29) is an aromatic heterocyclic organic compound similar to pyridine. The systematic study of pyrimidines was carried out and named ‘pyrimidin’ by a German chemist, Adolf Pinner (1885).
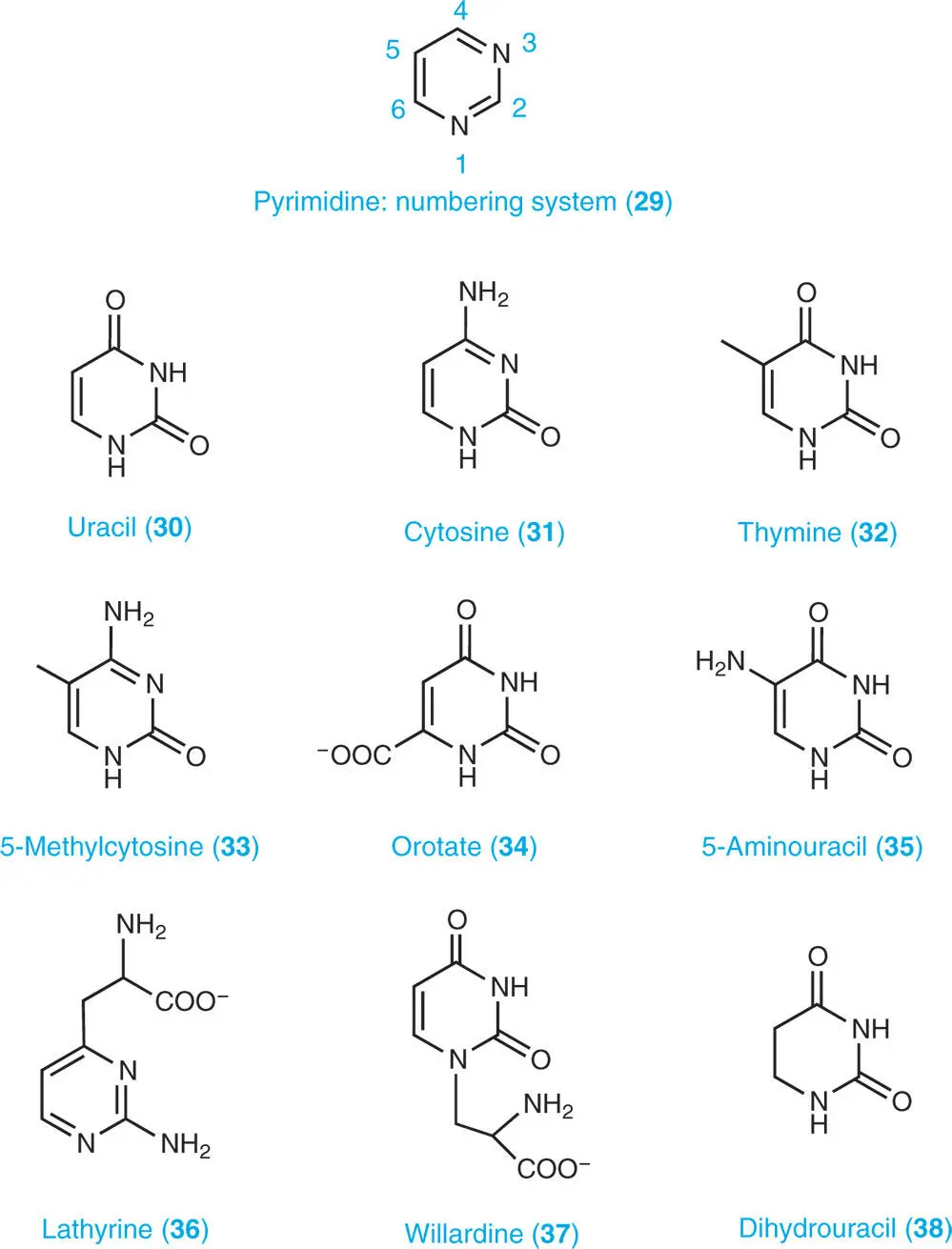
Uracil ( 30), cytosine ( 31), and thymine ( 32) are the common nucleobases of nucleic acids. DNA and RNA also contain other bases that have been modified after formation of the nucleic acid chain. In the case of DNA, the most common modified base is 5-methylcytosine ( 33).
Orotate (pyrimidine carboxylic acid) ( 34) is an intermediate of the de novo pyrimidine biosynthesis. A number of secondary products, such as 5-aminouracil ( 35), lathyrine ( 36), and willardine ( 37) occur in plants (see Part VIII). Dihydrouracil ( 38) is an intermediate of uracil catabolism (see Part III).
Читать дальше

















