1.3.2.2 Pyrimidine Nucleosides
A pyrimidine nucleoside consists of a pyrimidine base and a five-carbon sugar, either ribose or deoxyribose. Uridine ( 39) and cytidine ( 40) are produced as catabolites of pyrimidine ribonucleotides and RNA. Thymidine ( 41) is a catabolite of DNA. There are many modified bases in RNA, including those contained in the nucleosides, pseudouridine (5-ribosyluracil) ( 42), and dihydrouridine ( 43). Pseudouridine is an isomer of the nucleoside uridine ( 39) in which the uracil is attached via a carbon–carbon linkage instead of a nitrogen–carbon glycosidic bond. It is the most prevalent of the over 100 different modified nucleosides found in RNA.
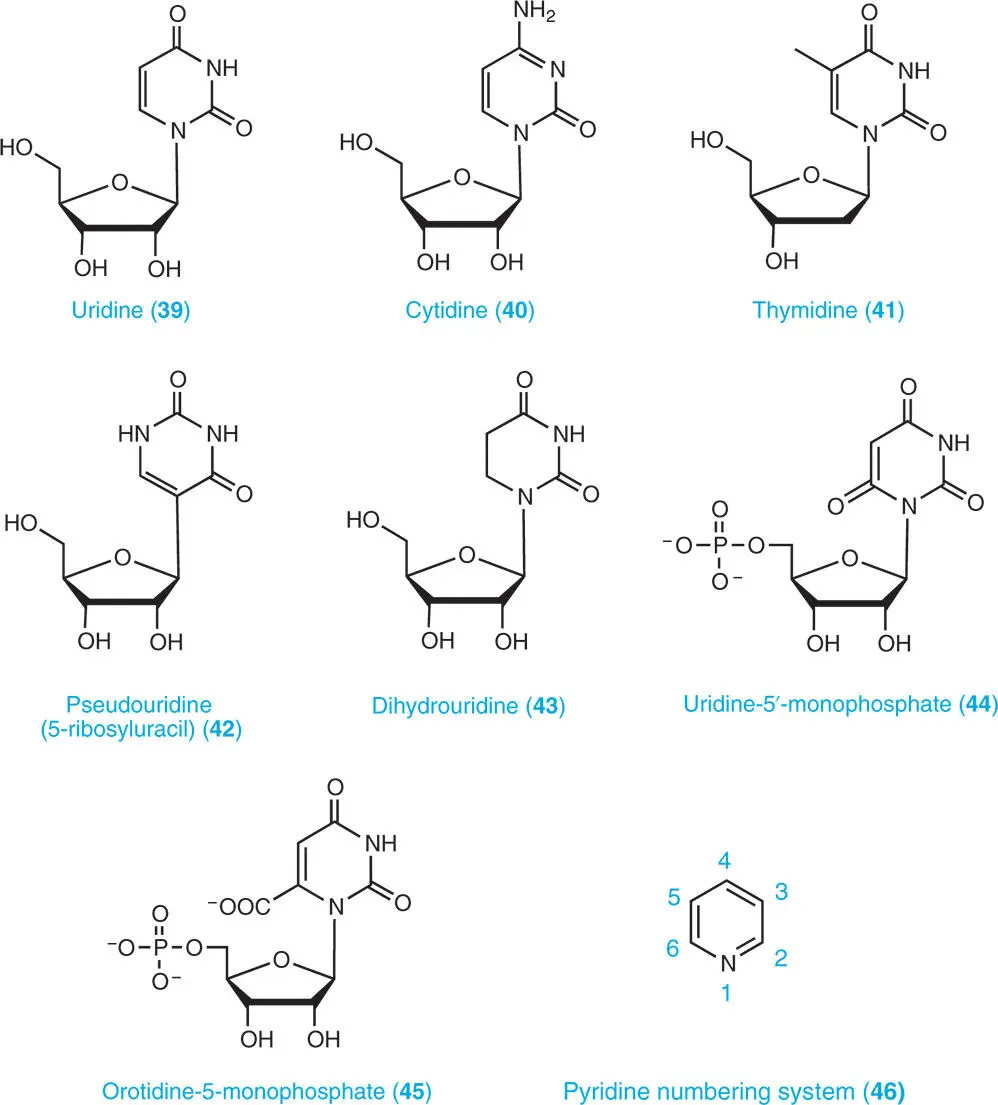
1.3.2.3 Pyrimidine Nucleotides
A pyrimidine nucleotide is composed of a pyrimidine base, a sugar (ribose or deoxyribose) and at least a phosphate group. Examples of different forms of pyrimidine nucleotide structures are uridine-5′-monophosphate (5′-UMP usually abbreviated as UMP) ( 44) and orotidine-5′-monophosphate (5′-OMP) ( 45), an intermediate of the de novo pyrimidine biosynthesis.
Pyridine ( 46) is a basic heterocyclic organic compound. It is structurally related to benzene, with one methine group replaced by a nitrogen atom, and was discovered in 1849 by a Scottish chemist, Thomas Anderson, as one of the constituents of bone oil. Pyridine-related compounds include the catabolites nicotinate ( 47) and nicotinamide ( 48), and quinolinate ( 49) and NMN ( 50), which are intermediates of the biosynthesis of NAD ( 51) and NADP. These compounds act as common coenzymes involved in many redox reactions, carrying electrons from one reaction to another in all living cells. Each coenzyme consists of pyridine purine nucleotides joined through their phosphate groups. NAD(P) exists in two forms: an oxidized and reduced form abbreviated as NAD(P) +and NAD(P)H respectively.
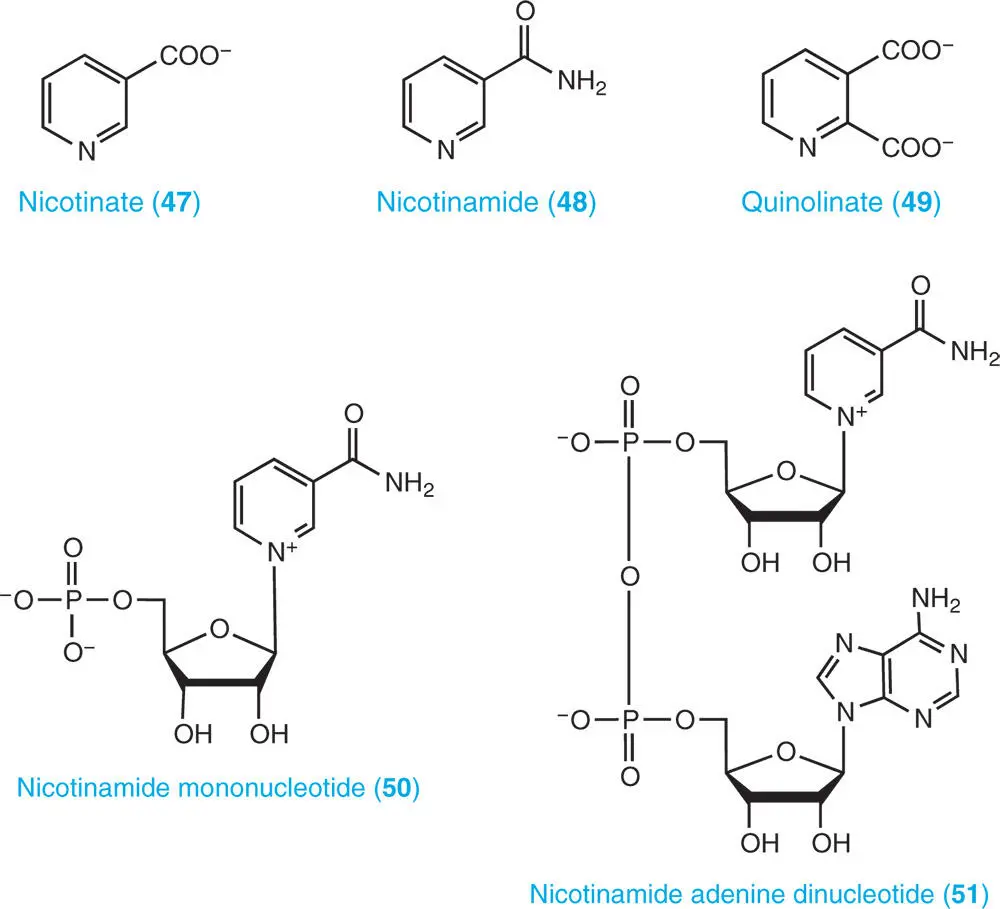
Nomenclature and abbreviations of nucleotide-related compounds and major chemical structures of purines, pyrimidine, and pyridine are described.
1 Ashihara, H., Yokota, T., and Crozier, A. (2013). Purine alkaloids, cytokinins, and purine-like neurotoxin alkaloids. In: Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes (eds. K.G. Ramawat and J.-M. Mérillon), 953–975. Berlin, Heidelberg: Springer.
2 Boniecka, J., Prusińska, J., Dąbrowska, G.B., and Goc, A. (2017). Within and beyond the stringent response-RSH and (p)ppGpp in plants. Planta 246: 817–842.
3 Burnstock, G. and Verkhratsky, A. (2012). Early history of purinergic signalling. In: Purinergic Signalling and the Nervous System, 7–66. Berlin, Heidelberg: Springer.
4 Fischer, E. (1884). Ueber die Harnsäure. I. Ber. Dtsch. Chem. Ges. 17: 328–338.
5 Harden, A. and Young, W.J. (1906). The alcoholic ferment of yeast-juice. Part II.—The coferment of yeast-juice. Proc. R. Soc. London, Ser. B 78: 369–375.
6 Henderson, J.F. and Paterson, A.R.P. (1973). Nucleotide Metabolism - an Introduction. New York: Academic Press.
7 IUPAC-IUB Commission on Biochemical Nomenclature (1970). Abbreviations and symbols for nucleic acids, polynucleotides and their constituents. Recommendations 1970. J. Biol. Chem. 245: 5171–5176.
8 Pietrowska-Borek, M., Nuc, K., Zielezińska, M., and Guranowski, A. (2011). Diadenosine polyphosphates (Ap3A and Ap4A) behave as alarmones triggering the synthesis of enzymes of the phenylpropanoid pathway in Arabidopsis thaliana. FEBS Open Bio 1: 1–6.
9 Pinner, A. (1885). Ueber die Einwirkung von Acetessigäther auf die Amidine Pyrimidin. Ber. Dtsch. Chem. Ges. A18: 759–760.
2 Occurrence of Nucleotides and Related Metabolites in Plants
2.1 Purines and Pyrimidines
Purines and pyrimidines are major chemical constituents of cells and occur primarily as components of DNA and RNA (polymerized nucleotides), and to a much lesser extent in the form of ‘free’ (aka ‘soluble’) nucleotides. Compared with nucleotides, free nucleosides and bases usually represent a very small fraction of the total purine and pyrimidine content of living cells, including plant cells. However, there are exceptions to this generalization, including the occurrence of substantial amounts caffeine ( 1) in plants such as coffee ( Coffea arabica and Coffea robusta ) and tea ( Camellia sinensis ), and theobromine ( 2) in cocoa ( Theobroma cocao ). Although not found in plants, the arabinosides of both thymine ( 3) and uracil ( 4) have been detected in the Caribbean sponge, Cryptotethya crypta . Guanine, in the silvery constituent of fish scales, and the excretion product, uric acid, are both free purines found in animal cells, but only occur in substantial amounts extracellularly (Henderson and Paterson 1973).
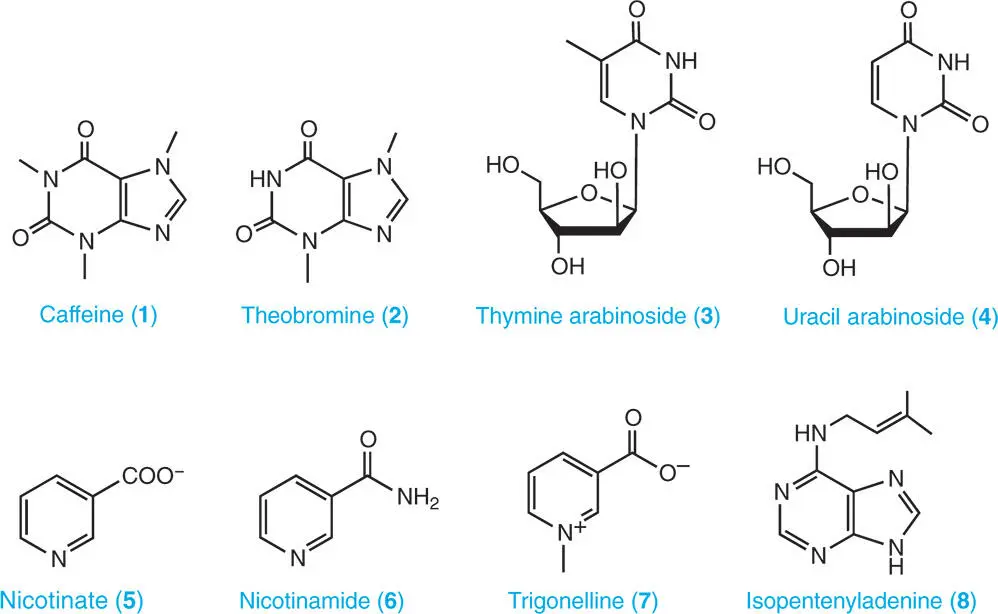
2.1.1 Concentration of Purine and Pyrimidine Nucleotides
Plants contain complex mixtures of free nucleotides. The principal components of such mixtures can be isolated and determined using HPLC combined with absorbance or, preferably, mass spectrometric detection. However, the extraction of nucleotides from cells is a crucial step. With some plant tissues, the extraction procedure has a profound influence on the resultant analytical profile, and special precautions must be taken if the results are to truly represent the nucleotide composition of living cells. Because of the presence of enzymes that hydrolyse nucleotides and nucleosides, such as non-specific acid phosphatases and nucleosidases, extraction of nucleotide-related compounds from plant material is extremely challenging. These hydrolytic enzymes are not denatured during extraction and remain active even in cold methanolic extracts.
Free nucleotides are separated from cellular macromolecular components by cold extraction procedures in which the macromolecules are precipitated; the usual extraction solvents are dilute perchloric acid or trichloroacetic acid. Prior to analysis, perchloric acid extracts are neutralized with KOH, precipitating KClO 4, while those treated with trichloroacetic acid are partitioned against diethyl ether to remove the acid. Usually, the concentration of nucleotides is low, so the aqueous fraction is lyophilized, and redissolved in small amounts of an aqueous solvent prior to HPLC.
Some examples of the levels of purine and pyrimidine nucleotide levels in cultured plant cells and leaves are shown in Table 2.1. The results were obtained with cultured cells of Arabidopsis thaliana (thale cress), Nicotiana tabacum (tobacco), Lycopersicon esculentum (tomato), and Catharanthus roseus (Madagascar periwinkle) at the exponential stage of growth. For comparison, nucleotide levels of cultured C. roseus cells grown in a phosphate (Pi)-deficient medium are also shown. Table 2.1also lists the levels in young leaves of A. thaliana , N. tabacum , L. esculentum and Ca. sinensis .
Читать дальше














