(1.21) 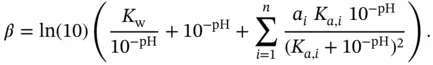
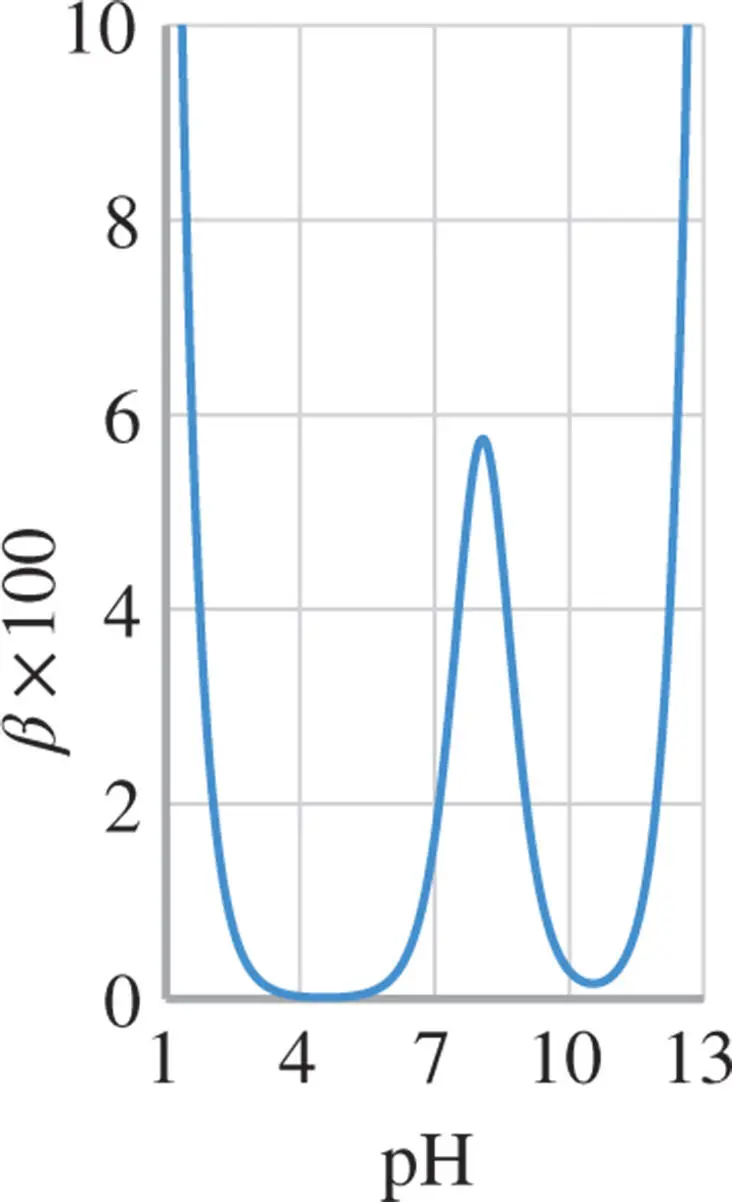
Figure 1.7 Buffer capacity of 0.1 M Tris (p  ) in an aqueous solution.
) in an aqueous solution.
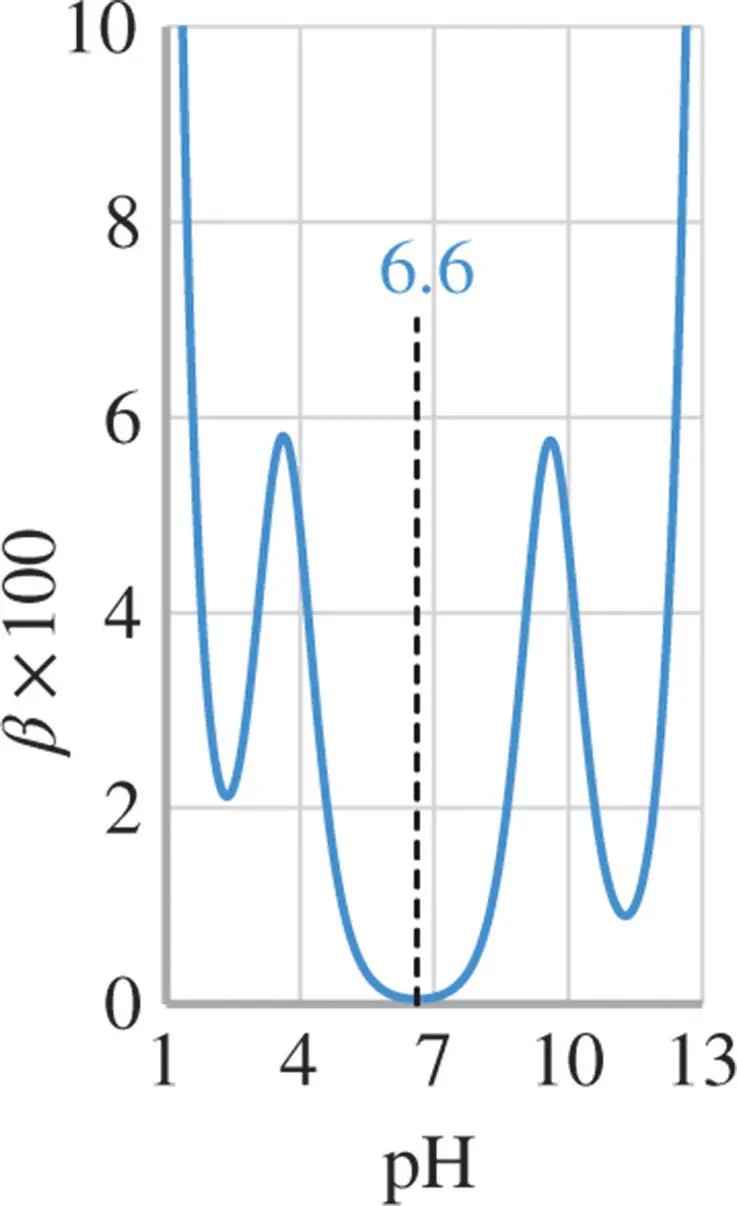
Figure 1.8 Buffer capacity of 0.1 M  -alanine (p
-alanine (p  and 9.6) in an aqueous solution. The buffer capacity at the isoelectric point (
and 9.6) in an aqueous solution. The buffer capacity at the isoelectric point ( 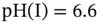 ) is low.
) is low.
In this case the index 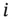 refers to each weak acid (or weak base) or each functional group if the same molecule carries weak acid groups and weak basic groups. Figures 1.8and 1.9were plotted using this equation.
refers to each weak acid (or weak base) or each functional group if the same molecule carries weak acid groups and weak basic groups. Figures 1.8and 1.9were plotted using this equation.
Figure 1.8shows the buffer capacity of a 0.1 M solution of 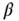 -alanine as a function of pH and Figure 1.9for a 0.1 M glycyl-aspartic acid. Note that both
-alanine as a function of pH and Figure 1.9for a 0.1 M glycyl-aspartic acid. Note that both 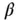 -alanine and glycyl-aspartic acid do have isoelectric points (IEP), however only glycyl-aspartic acid has a good buffer capacity around its or close to the pH(I). A high buffer capacity at or close to the pH(I) is a highly desired property in the ESTs, as it allows the pH to be stabilized with minimal increase in the conductivity.
-alanine and glycyl-aspartic acid do have isoelectric points (IEP), however only glycyl-aspartic acid has a good buffer capacity around its or close to the pH(I). A high buffer capacity at or close to the pH(I) is a highly desired property in the ESTs, as it allows the pH to be stabilized with minimal increase in the conductivity.
The total conductivity of the buffer must be higher than or close to the conductivity of the sample, otherwise it causes band destacking (Section 2.14). On the other hand, the lower the conductivity of the buffering electrolyte to the total conductivity of the buffer solution the better, as some room is left for the addition of some electrolytes with the same mobility as the analytes. This is important for the production of symmetric peaks in most of the separation modes. Isotachophoresis and isoelectric focusing are exceptions to this rule as they are based on special separation mechanisms. Some samples do have low conductivities, in this case the development of buffer solutions with low conductivity, i.e. designed for high field strengths operations, is always an active topic of research, [12] as they lead to high separation efficiencies (Section 3.5).
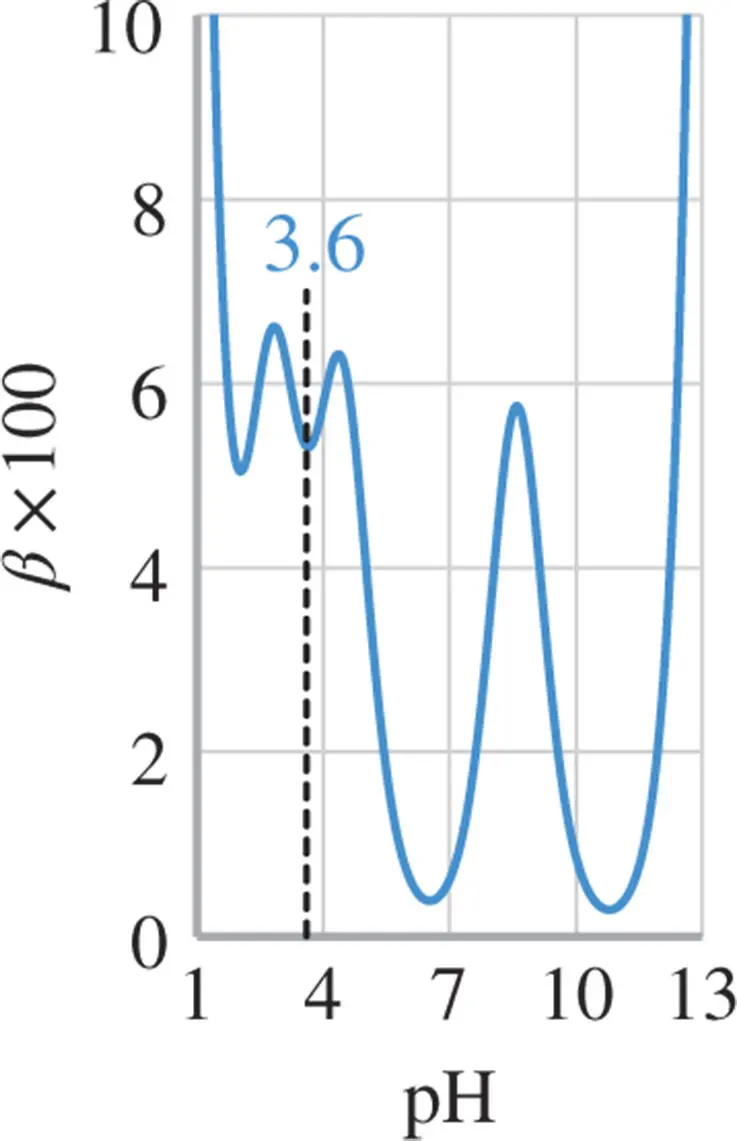
Figure 1.9 Buffer capacity of 0.1 M glycyl-aspartic acid (p  , 4.45 and 8.6) in an aqueous solution. Note the high buffer capacity at
, 4.45 and 8.6) in an aqueous solution. Note the high buffer capacity at 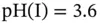 .
.
Another very useful concept is the normalized buffering capacity/conductivity ratio .[13] Here, the buffering capacity at each pH is divided by the buffer conductivity at this pH. A high buffer capacity and low buffer conductivity are highly desirable. This allows different buffers to be compared among themselves at different pHs. Such performance rankings of good buffers are still missing in the literature.
1.2 Binary Mixtures and Other Solvents
Besides pure water, some binary mixtures of water and a protogenic organic polar solvent (methanol, ethanol or 2-propanol) or a protophilic organic polar solvent (acetonitrile, acetone or dimethylsulfoxide) have been studied and applied as solvents in ESTs [14,15], over the years. For instance, water-methanol, [16] water-ethanol, [17] water-isopropanol, [18] and water-acetonitrile. [19] For some applications they are necessary for many reasons. Firstly, to improve the solubility of analytes that exhibit poor solubility in pure water. Secondly, to control the electroosmotic flow in order to shorten or extend the run time. Apparent mobility (from injection point to detection point) can be increased for analytes that are easily separated, shortening the run time. This decreases the distance run by the analytes in the reference frame of the liquid phase, providing the benefit of a shorter run time but consequently decreasing the separation efficiency. Many mixtures are difficult to separate and in this case the electroosmotic flow is set to run in the opposite direction to the analytes. This means that the analytes now run longer distances in the reference frame of the liquid, which means that the separation efficiency is tremendously increased. However, as discussed in many Sections (e.g. 2.9 and 2.10), electroosmosis is difficult to control and exhibits poor repeatability from run to run. This has a negative impact on peak identification and quantitative analyses. Third, a number of other minor benefits occur depending on the amount of organic modifier present; these benefits include: many binary mixtures exhibit a much longer shelf life (microbial growth is inhibited); some can be stored at  8
8  C without freezing, so that they reach working temperature much quicker than frozen solutions; standard solutions of biomolecules last much longer in these mixtures; and the dynamic viscosity of the mixture can be increased/decreased, which is beneficial when working with micro-channels and capillaries with a broad range of diameters.
C without freezing, so that they reach working temperature much quicker than frozen solutions; standard solutions of biomolecules last much longer in these mixtures; and the dynamic viscosity of the mixture can be increased/decreased, which is beneficial when working with micro-channels and capillaries with a broad range of diameters.
Finally, there are a few applications (for instance the separation of aminium ions, quaternary ammonium ions, and coordination complexes) that can be run with pure propylene carbonate, formamide or other solvents, since the analytes remain charged within them. Moreover, propylene carbonate, for instance, does not produce gases at the electrodes, allowing the separation to be run in sealed systems.
1 1 Reif, F. (1965). Fundamentals of Statistical and Thermal Physics. Tokyo: McGraw-Hill Kogakusha.
2 2 Rahm, M., Zeng, T., and Hoffmann, R. (2019). Journal of the American Chemical Society 141: 342–351.
3 3 Wohlfart, C. and Lechner, M.D. (eds.) (2008). Static Dielectric Constants of Pure Liquids and Binary Liquid Mixtures, Landolt–Börnstein. Berlin: Springer-Verlag.
4 4 Soustelle, M. (2016). Ionic and Electrochemical Equilibria. Hoboken, NJ: Wiley.
5 5 Franks, F. (1979). Water: A Comprehensive Treatise: Recent Advances, vol. 6. Boston, MA: Springer-Verlag.
Читать дальше

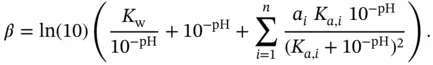

 ) in an aqueous solution.
) in an aqueous solution.
 -alanine (p
-alanine (p  and 9.6) in an aqueous solution. The buffer capacity at the isoelectric point (
and 9.6) in an aqueous solution. The buffer capacity at the isoelectric point ( 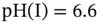 ) is low.
) is low.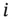 refers to each weak acid (or weak base) or each functional group if the same molecule carries weak acid groups and weak basic groups. Figures 1.8and 1.9were plotted using this equation.
refers to each weak acid (or weak base) or each functional group if the same molecule carries weak acid groups and weak basic groups. Figures 1.8and 1.9were plotted using this equation.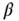 -alanine as a function of pH and Figure 1.9for a 0.1 M glycyl-aspartic acid. Note that both
-alanine as a function of pH and Figure 1.9for a 0.1 M glycyl-aspartic acid. Note that both 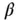 -alanine and glycyl-aspartic acid do have isoelectric points (IEP), however only glycyl-aspartic acid has a good buffer capacity around its or close to the pH(I). A high buffer capacity at or close to the pH(I) is a highly desired property in the ESTs, as it allows the pH to be stabilized with minimal increase in the conductivity.
-alanine and glycyl-aspartic acid do have isoelectric points (IEP), however only glycyl-aspartic acid has a good buffer capacity around its or close to the pH(I). A high buffer capacity at or close to the pH(I) is a highly desired property in the ESTs, as it allows the pH to be stabilized with minimal increase in the conductivity.
 , 4.45 and 8.6) in an aqueous solution. Note the high buffer capacity at
, 4.45 and 8.6) in an aqueous solution. Note the high buffer capacity at 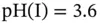 .
. 8
8  C without freezing, so that they reach working temperature much quicker than frozen solutions; standard solutions of biomolecules last much longer in these mixtures; and the dynamic viscosity of the mixture can be increased/decreased, which is beneficial when working with micro-channels and capillaries with a broad range of diameters.
C without freezing, so that they reach working temperature much quicker than frozen solutions; standard solutions of biomolecules last much longer in these mixtures; and the dynamic viscosity of the mixture can be increased/decreased, which is beneficial when working with micro-channels and capillaries with a broad range of diameters.










