1 ...6 7 8 10 11 12 ...15 (1.5) 
where the source of the OH −ions is the self-ionization of water. These can be seen as heterolytic substitution reactions ; note that both H 2O and OH −work as Brønsted bases in this case. For the ionization of amines (e.g., the secondary amine N-methylmethanamine) there are also at least two possible reactions that can occur:
(1.6) 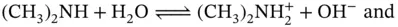
(1.7) 
Here, both H 2O and the H 3O +cation function as Brønsted acids. For a detailed discussion of dissociation and ionization in water see Soustelle (2016).[4]
Finally, it is important to remember that a small fraction of the self-ionization reactions of water produce H 5O 2 +and an even smaller fraction produce H 7O 3 +, as well as other ions.
A great deal of knowledge is continuously generated about the internal structure and dynamics of liquid water. Topics such as self-ionization of water, the ions produced, the average life-time of these ions, solvation, and water clusters are difficult to treat because they can not be treated as events in a continuous, homogeneous fluid. They should be treated within the many-body problem theories; however, the number of entities in the case of water must be great to be realistic because the water molecules are very sticky with each other due to hydrogen bonding. So one can always remember a saying that remains true: “Of all known liquids, water is probably the most studied and the least understood”.[5]
1.1.7 Hydrophilicity, Hydrophobicity, and LogP
Hydrophilicity and hydrophobicity are qualitative terms that refer to chemical substances that, respectively, dissolve in water (strong affinity for water) or in non-polar substances (weak affinity for water). Solvation and the formation of hydrogen bonds are important processes involved in the dissolution of hydrophilic solutes in water (an environment rich in hydrogen bonds). On the other hand, no solvation or hydrogen bond formation occurs when attempting to dissolve hydrophobic (non-polar) substances in water. The dissolution of non-polar substances in non-polar solvents occurs because the positive entrophy change of the system (solvent  solute) and the action of van der Waals forces among the hydrophobic solutes and the non-polar solvents. Note that van der Waals forces are much weaker than hydrogen bonds.
solute) and the action of van der Waals forces among the hydrophobic solutes and the non-polar solvents. Note that van der Waals forces are much weaker than hydrogen bonds.
Microdrops of hydrophobic substances (e.g., vegetable oil) that are dispersed in water tend to irreversibly join (fuse) when random Brownian motion causes them to collide with each other. Each fusion event causes a sudden increase in the total number of hydrogen bonds in the given volume of water. Macrodrops are formed and at the end expelled from the bulk of the water, which again suddenly increases the total number of hydrogen bonds among water molecules. Note that the inverse reaction (converting a macrodrop of oil into microdroplets) is only possible with the input of a significant amount of energy (usually from vigorous mechanical stirring), as a large quantity of hydrogen bonds must be broken. Thus, it would be fairer to call water oleophobic instead of calling non-polar substances hydrophobic .
LogP (or CLOGP) is a well defined quantitative parameter that is related to the hydrophilicity and hydrophobicity of a chemical substance. It is a real number that goes from minus infinity to plus infinity ( 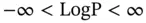 ) and is considered a fundamental parameter in ESTs and in many other fields (e.g., organic chemistry and pharmaceutical sciences).
) and is considered a fundamental parameter in ESTs and in many other fields (e.g., organic chemistry and pharmaceutical sciences).
Table 1.2 Examples of some LogP values. These are the average values of the  most representative items of each data set. Measurements were taken at room temperature using pure water. The only exception was L-nicotine, which was measured at pH 10.3.
most representative items of each data set. Measurements were taken at room temperature using pure water. The only exception was L-nicotine, which was measured at pH 10.3.
(Source: Data from DDBST GmbH, Oldenburg, Germany, www.ddbst.com.).
| Analyte |
LogP |
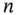 |
| Acetaldehyde |
0.43 |
1 |
| Acrylamide |
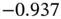 |
3 |
| Aniline |
0.975 |
16 |
| Benzo[a]pyrene |
6.898 |
11 |
| Caffeine |
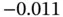 |
4 |
| Dimethylnitrosamine |
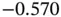 |
2 |
| L-nicotine |
1.39 |
1 |
The LogP of a chemical substance is defined as the decadic logarithm of its 1-octanol/water partition coefficient. The substance is dissolved in water or 1-octanol and then equal volumes of these solvents (one of which contains the substance under analysis) are placed in contact with each other and left to reach equilibrium. When equilibrium is reached the value of 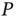 is calculated using the following equations:
is calculated using the following equations:
(1.8) 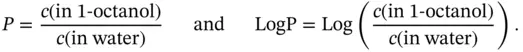
Table 1.2gives the LogP values of a few chemical substances. Such values are already used in structure–property correlations and quantitative studies of structure–activity relationships. As more LogP values become available, they will be used in pharmaceutical sciences, pharmanalysis, biochemistry, and analytical chemistry more frequently.
1.1.8 Gibbs Free Energy Change
The solubility (due to dissolution and/or dissociation and/or ionization) of a specific quantity of solute in a certain volume of a given solvent at temperature 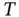 can be predicted by calculating the Gibbs free energy change:
can be predicted by calculating the Gibbs free energy change: 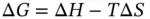 . The temperature and changes of enthalpy (
. The temperature and changes of enthalpy ( 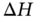 ) and entropy (
) and entropy ( 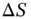 ) all affect the solubility of a solute. The Gibbs free energy change mathematically expresses the role of each of these three thermodynamic variables within reactions (including dissolution). Regardless of the type of reaction (a dissolution, a dissolution with dissociation or a dissolution with ionization), all chemical reactions follow the rule: if
) all affect the solubility of a solute. The Gibbs free energy change mathematically expresses the role of each of these three thermodynamic variables within reactions (including dissolution). Regardless of the type of reaction (a dissolution, a dissolution with dissociation or a dissolution with ionization), all chemical reactions follow the rule: if 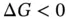 for a given amount of solute and solvent then the reaction occurs (the solute is soluble), although the velocity may be slow and depends on the potential barriers, but if
for a given amount of solute and solvent then the reaction occurs (the solute is soluble), although the velocity may be slow and depends on the potential barriers, but if 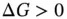 then the reaction will not occur (the specified quantity of solute is not soluble). Generally it is easy to estimate
then the reaction will not occur (the specified quantity of solute is not soluble). Generally it is easy to estimate 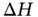 ; however, it is very difficult to evaluate
; however, it is very difficult to evaluate 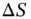 in most real situations. Dissociation of a salt, for instance, increases the number of states available to the sub-system salt, as solvation occurs, but decreases the number of states available to the sub-system water molecules. This works as a trade off: as the entropy of the salt ions increases the entropy of the water molecules is consequently decreased. When the overall entropy change is negative then lower temperatures favor the occurrence of the dissolution. By contrast, if the entropy change is positive then higher temperatures will favor dissolution. It is necessary to note that lower temperatures generally decrease the velocity of reactions, since a lower thermal energy decreases the probability of reactants overcoming the energy potential barriers of the intermediate products. Remember that entropy is proportional to the logarithm of the number of states available to the system, within the constrains (volume and total energy) imposed by the system. A didactic explanation of enthalpy, entropy, and Gibbs free energy is given by Connors (2002) [6] and a quantitatively rigorous approach is described by Reif (1965) [1]. The above presented theory is also valid for the solubilization processes of hydrophobic solutes (and colloids) in aqueous solutions with the aid of micelles (surfactants) or microemulsions (stabilized submicron droplets of oil). The same can be told with the inverse, the solubilization of hydrophilic solutes in hydrophobic solvents.
in most real situations. Dissociation of a salt, for instance, increases the number of states available to the sub-system salt, as solvation occurs, but decreases the number of states available to the sub-system water molecules. This works as a trade off: as the entropy of the salt ions increases the entropy of the water molecules is consequently decreased. When the overall entropy change is negative then lower temperatures favor the occurrence of the dissolution. By contrast, if the entropy change is positive then higher temperatures will favor dissolution. It is necessary to note that lower temperatures generally decrease the velocity of reactions, since a lower thermal energy decreases the probability of reactants overcoming the energy potential barriers of the intermediate products. Remember that entropy is proportional to the logarithm of the number of states available to the system, within the constrains (volume and total energy) imposed by the system. A didactic explanation of enthalpy, entropy, and Gibbs free energy is given by Connors (2002) [6] and a quantitatively rigorous approach is described by Reif (1965) [1]. The above presented theory is also valid for the solubilization processes of hydrophobic solutes (and colloids) in aqueous solutions with the aid of micelles (surfactants) or microemulsions (stabilized submicron droplets of oil). The same can be told with the inverse, the solubilization of hydrophilic solutes in hydrophobic solvents.
Читать дальше


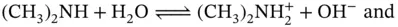

 solute) and the action of van der Waals forces among the hydrophobic solutes and the non-polar solvents. Note that van der Waals forces are much weaker than hydrogen bonds.
solute) and the action of van der Waals forces among the hydrophobic solutes and the non-polar solvents. Note that van der Waals forces are much weaker than hydrogen bonds.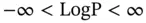 ) and is considered a fundamental parameter in ESTs and in many other fields (e.g., organic chemistry and pharmaceutical sciences).
) and is considered a fundamental parameter in ESTs and in many other fields (e.g., organic chemistry and pharmaceutical sciences). most representative items of each data set. Measurements were taken at room temperature using pure water. The only exception was L-nicotine, which was measured at pH 10.3.
most representative items of each data set. Measurements were taken at room temperature using pure water. The only exception was L-nicotine, which was measured at pH 10.3.
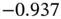
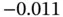
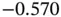
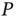 is calculated using the following equations:
is calculated using the following equations: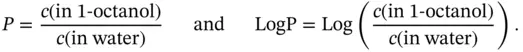
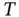 can be predicted by calculating the Gibbs free energy change:
can be predicted by calculating the Gibbs free energy change: 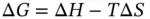 . The temperature and changes of enthalpy (
. The temperature and changes of enthalpy ( 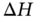 ) and entropy (
) and entropy ( 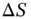 ) all affect the solubility of a solute. The Gibbs free energy change mathematically expresses the role of each of these three thermodynamic variables within reactions (including dissolution). Regardless of the type of reaction (a dissolution, a dissolution with dissociation or a dissolution with ionization), all chemical reactions follow the rule: if
) all affect the solubility of a solute. The Gibbs free energy change mathematically expresses the role of each of these three thermodynamic variables within reactions (including dissolution). Regardless of the type of reaction (a dissolution, a dissolution with dissociation or a dissolution with ionization), all chemical reactions follow the rule: if 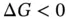 for a given amount of solute and solvent then the reaction occurs (the solute is soluble), although the velocity may be slow and depends on the potential barriers, but if
for a given amount of solute and solvent then the reaction occurs (the solute is soluble), although the velocity may be slow and depends on the potential barriers, but if 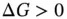 then the reaction will not occur (the specified quantity of solute is not soluble). Generally it is easy to estimate
then the reaction will not occur (the specified quantity of solute is not soluble). Generally it is easy to estimate 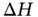 ; however, it is very difficult to evaluate
; however, it is very difficult to evaluate 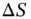 in most real situations. Dissociation of a salt, for instance, increases the number of states available to the sub-system salt, as solvation occurs, but decreases the number of states available to the sub-system water molecules. This works as a trade off: as the entropy of the salt ions increases the entropy of the water molecules is consequently decreased. When the overall entropy change is negative then lower temperatures favor the occurrence of the dissolution. By contrast, if the entropy change is positive then higher temperatures will favor dissolution. It is necessary to note that lower temperatures generally decrease the velocity of reactions, since a lower thermal energy decreases the probability of reactants overcoming the energy potential barriers of the intermediate products. Remember that entropy is proportional to the logarithm of the number of states available to the system, within the constrains (volume and total energy) imposed by the system. A didactic explanation of enthalpy, entropy, and Gibbs free energy is given by Connors (2002) [6] and a quantitatively rigorous approach is described by Reif (1965) [1]. The above presented theory is also valid for the solubilization processes of hydrophobic solutes (and colloids) in aqueous solutions with the aid of micelles (surfactants) or microemulsions (stabilized submicron droplets of oil). The same can be told with the inverse, the solubilization of hydrophilic solutes in hydrophobic solvents.
in most real situations. Dissociation of a salt, for instance, increases the number of states available to the sub-system salt, as solvation occurs, but decreases the number of states available to the sub-system water molecules. This works as a trade off: as the entropy of the salt ions increases the entropy of the water molecules is consequently decreased. When the overall entropy change is negative then lower temperatures favor the occurrence of the dissolution. By contrast, if the entropy change is positive then higher temperatures will favor dissolution. It is necessary to note that lower temperatures generally decrease the velocity of reactions, since a lower thermal energy decreases the probability of reactants overcoming the energy potential barriers of the intermediate products. Remember that entropy is proportional to the logarithm of the number of states available to the system, within the constrains (volume and total energy) imposed by the system. A didactic explanation of enthalpy, entropy, and Gibbs free energy is given by Connors (2002) [6] and a quantitatively rigorous approach is described by Reif (1965) [1]. The above presented theory is also valid for the solubilization processes of hydrophobic solutes (and colloids) in aqueous solutions with the aid of micelles (surfactants) or microemulsions (stabilized submicron droplets of oil). The same can be told with the inverse, the solubilization of hydrophilic solutes in hydrophobic solvents.










